Alterations in vasodilator-stimulated phosphoprotein (VASP) phosphorylation: associations with asthmatic phenotype, airway inflammation and beta2-agonist use
- PMID: 16480498
- PMCID: PMC1388207
- DOI: 10.1186/1465-9921-7-25
Alterations in vasodilator-stimulated phosphoprotein (VASP) phosphorylation: associations with asthmatic phenotype, airway inflammation and beta2-agonist use
Abstract
Background: Vasodilator-stimulated phosphoprotein (VASP) mediates focal adhesion, actin filament binding and polymerization in a variety of cells, thereby inhibiting cell movement. Phosphorylation of VASP via cAMP and cGMP dependent protein kinases releases this "brake" on cell motility. Thus, phosphorylation of VASP may be necessary for epithelial cell repair of damage from allergen-induced inflammation. Two hypotheses were examined: (1) injury from segmental allergen challenge increases VASP phosphorylation in airway epithelium in asthmatic but not nonasthmatic normal subjects, (2) regular in vivo beta2-agonist use increases VASP phosphorylation in asthmatic epithelium, altering cell adhesion.
Methods: Bronchial epithelium was obtained from asthmatic and non-asthmatic normal subjects before and after segmental allergen challenge, and after regularly inhaled albuterol, in three separate protocols. VASP phosphorylation was examined in Western blots of epithelial samples. DNA was obtained for beta2-adrenergic receptor haplotype determination.
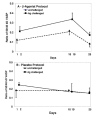
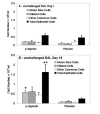
Results: Although VASP phosphorylation increased, it was not significantly greater after allergen challenge in asthmatics or normals. However, VASP phosphorylation in epithelium of nonasthmatic normal subjects was double that observed in asthmatic subjects, both at baseline and after challenge. Regularly inhaled albuterol significantly increased VASP phosphorylation in asthmatic subjects in both unchallenged and antigen challenged lung segment epithelium. There was also a significant increase in epithelial cells in the bronchoalveolar lavage of the unchallenged lung segment after regular inhalation of albuterol but not of placebo. The haplotypes of the beta2-adrenergic receptor did not appear to associate with increased or decreased phosphorylation of VASP.
Conclusion: Decreased VASP phosphorylation was observed in epithelial cells of asthmatics compared to nonasthmatic normals, despite response to beta-agonist. The decreased phosphorylation does not appear to be associated with a particular beta2-adrenergic receptor haplotype. The observed decrease in VASP phosphorylation suggests greater inhibition of actin reorganization which is necessary for altering attachment and migration required during epithelial repair.
Figures


Similar articles
-
β2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein.Am J Respir Cell Mol Biol. 2012 Jan;46(1):48-54. doi: 10.1165/rcmb.2011-0217OC. Am J Respir Cell Mol Biol. 2012. PMID: 22210825 Free PMC article.
-
Regulation of VASP phosphorylation in cardiac myocytes: differential regulation by cyclic nucleotides and modulation of protein expression in diabetic and hypertrophic heart.Am J Physiol Heart Circ Physiol. 2009 Nov;297(5):H1697-710. doi: 10.1152/ajpheart.00595.2009. Epub 2009 Sep 4. Am J Physiol Heart Circ Physiol. 2009. PMID: 19734360 Free PMC article.
-
Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism.J Biol Chem. 2015 May 1;290(18):11403-16. doi: 10.1074/jbc.M115.645788. Epub 2015 Mar 10. J Biol Chem. 2015. PMID: 25759389 Free PMC article.
-
Inhaled beta2-agonists and airway responses to allergen.J Allergy Clin Immunol. 1998 Nov;102(5):S96-9. doi: 10.1016/s0091-6749(98)70038-7. J Allergy Clin Immunol. 1998. PMID: 9819316 Review.
-
Modulation of vasodilator-stimulated phosphoprotein in vivo in human epithelial cells by segmental allergen challenge and beta2-agonist therapy.Chest. 2003 Mar;123(3 Suppl):377S. Chest. 2003. PMID: 12628994 Review. No abstract available.
Cited by
-
The enabled homolog gene polymorphisms are associated with susceptibility and progression of childhood IgA nephropathy.Exp Mol Med. 2009 Nov 30;41(11):793-801. doi: 10.3858/emm.2009.41.11.085. Exp Mol Med. 2009. PMID: 19641378 Free PMC article.
-
Protective Effect of Quercetin in LPS-Induced Murine Acute Lung Injury Mediated by cAMP-Epac Pathway.Inflammation. 2018 Jun;41(3):1093-1103. doi: 10.1007/s10753-018-0761-3. Inflammation. 2018. PMID: 29569077
-
β2-adrenergic receptor agonists modulate human airway smooth muscle cell migration via vasodilator-stimulated phosphoprotein.Am J Respir Cell Mol Biol. 2012 Jan;46(1):48-54. doi: 10.1165/rcmb.2011-0217OC. Am J Respir Cell Mol Biol. 2012. PMID: 22210825 Free PMC article.
-
Proteome from patients with metabolic syndrome is regulated by quantity and quality of dietary lipids.BMC Genomics. 2015 Jul 8;16(1):509. doi: 10.1186/s12864-015-1725-8. BMC Genomics. 2015. PMID: 26152126 Free PMC article. Clinical Trial.
-
Role of Corneal Stromal Cells on Epithelial Cell Function during Wound Healing.Int J Mol Sci. 2018 Feb 4;19(2):464. doi: 10.3390/ijms19020464. Int J Mol Sci. 2018. PMID: 29401709 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

