The Ras-GRF1 exchange factor coordinates activation of H-Ras and Rac1 to control neuronal morphology
- PMID: 16481401
- PMCID: PMC1446076
- DOI: 10.1091/mbc.e05-10-0913
The Ras-GRF1 exchange factor coordinates activation of H-Ras and Rac1 to control neuronal morphology
Abstract
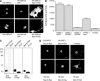
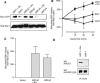
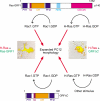
The Ras-GRF1 exchange factor has regulated guanine nucleotide exchange factor (GEF) activity for H-Ras and Rac1 through separate domains. Both H-Ras and Rac1 activation have been linked to synaptic plasticity and thus could contribute to the function of Ras-GRF1 in neuronal signal transduction pathways that underlie learning and memory. We defined the effects of Ras-GRF1 and truncation mutants that include only one of its GEF activities on the morphology of PC12 phaeochromocytoma cells. Ras-GRF1 required coexpression of H-Ras to induce morphological effects. Ras-GRF1 plus H-Ras induced a novel, expanded morphology in PC12 cells, which was characterized by a 10-fold increase in soma size and by neurite extension. A truncation mutant of Ras-GRF1 that included the Ras GEF domain, GRFdeltaN, plus H-Ras produced neurite extensions, but did not expand the soma. This neurite extension was blocked by inhibition of MAP kinase activation, but was independent of dominant-negative Rac1 or RhoA. A truncation mutant of Ras-GRF1 that included the Rac GEF domains, GRFdeltaC, produced the expanded phenotype in cotransfections with H-Ras. Cell expansion was inhibited by wortmannin or dominant-negative forms of Rac1 or Akt. GRFdeltaC binds H-Ras.GTP in both pulldown assays from bacterial lysates and by coimmunoprecipitation from HEK293 cells. These results suggest that coordinated activation of H-Ras and Rac1 by Ras-GRF1 may be a significant controller of neuronal cell size.
Figures





Similar articles
-
Ras guanine nucleotide releasing factor 1 (RasGrf1) enhancement of Trk receptor-mediated neurite outgrowth requires activation of both H-Ras and Rac.J Mol Neurosci. 2013 Jan;49(1):38-51. doi: 10.1007/s12031-012-9847-9. Epub 2012 Jun 29. J Mol Neurosci. 2013. PMID: 22744634
-
NGF-dependent formation of ruffles in PC12D cells required a different pathway from that for neurite outgrowth.Neurochem Int. 2007 Jul-Sep;51(2-4):216-26. doi: 10.1016/j.neuint.2007.04.032. Epub 2007 May 17. Neurochem Int. 2007. PMID: 17561310
-
p75-Ras-GRF1 is a c-Jun/AP-1 target protein: its up regulation results in increased Ras activity and is necessary for c-Jun-induced nonadherent growth of Rat1a cells.Mol Cell Biol. 2005 Apr;25(8):3324-37. doi: 10.1128/MCB.25.8.3324-3337.2005. Mol Cell Biol. 2005. PMID: 15798216 Free PMC article.
-
G protein beta gamma subunit-dependent Rac-guanine nucleotide exchange activity of Ras-GRF1/CDC25(Mm).Proc Natl Acad Sci U S A. 1999 Apr 27;96(9):4826-31. doi: 10.1073/pnas.96.9.4826. Proc Natl Acad Sci U S A. 1999. PMID: 10220378 Free PMC article.
-
Control of ras activation.Cancer Surv. 1996;27:87-100. Cancer Surv. 1996. PMID: 8909796 Review.
Cited by
-
Restoration of E-cadherin cell-cell junctions requires both expression of E-cadherin and suppression of ERK MAP kinase activation in Ras-transformed breast epithelial cells.Neoplasia. 2008 Dec;10(12):1444-58. doi: 10.1593/neo.08968. Neoplasia. 2008. PMID: 19048123 Free PMC article.
-
Ras guanine nucleotide releasing factor 1 (RasGrf1) enhancement of Trk receptor-mediated neurite outgrowth requires activation of both H-Ras and Rac.J Mol Neurosci. 2013 Jan;49(1):38-51. doi: 10.1007/s12031-012-9847-9. Epub 2012 Jun 29. J Mol Neurosci. 2013. PMID: 22744634
-
p21-Activated kinase 1 coordinates aberrant cell survival and pericellular proteolysis in a three-dimensional culture model for premalignant progression of human breast cancer.Neoplasia. 2008 Apr;10(4):314-29. doi: 10.1593/neo.07970. Neoplasia. 2008. PMID: 18392133 Free PMC article.
-
How to Target Activated Ras Proteins: Direct Inhibition vs. Induced Mislocalization.Mini Rev Med Chem. 2016;16(5):358-69. doi: 10.2174/1389557515666151001154002. Mini Rev Med Chem. 2016. PMID: 26423696 Free PMC article. Review.
-
Imprinted Rasgrf1 expression in neonatal mice affects olfactory learning and memory.Genes Brain Behav. 2011 Jun;10(4):392-403. doi: 10.1111/j.1601-183X.2011.00678.x. Epub 2011 Feb 10. Genes Brain Behav. 2011. PMID: 21251221 Free PMC article.
References
-
- Arendt, T. et al. (2004). Neuronal activation of Ras regulates synaptic connectivity. Eur. J. Neurosci. 19, 2953–2966. - PubMed
-
- Aznar, S., and Lacal, J. C. (2001). Rho signals to cell growth and apoptosis. Cancer Lett. 165, 1–10. - PubMed
-
- Baouz, S., Jacquet, E., Bernardi, A., and Parmeggiani, A. (1997). The N-terminal moiety of CDC25(Mm), a GDP/GTP exchange factor of Ras proteins, controls the activity of the catalytic domain. Modulation by calmodulin and calpain. J. Biol. Chem. 272, 6671–6676. - PubMed
-
- Bar-Sagi, D., and Feramisco, J. R. (1985). Microinjection of the Ras oncogene protein into PC12 cells induces morphological differentiation. Cell 42, 841–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

