Factors controlling fibroblast growth factor receptor-1's cytoplasmic trafficking and its regulation as revealed by FRAP analysis
- PMID: 16481405
- PMCID: PMC1446089
- DOI: 10.1091/mbc.e05-08-0749
Factors controlling fibroblast growth factor receptor-1's cytoplasmic trafficking and its regulation as revealed by FRAP analysis
Abstract
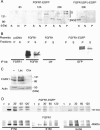
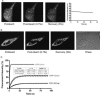
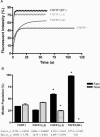
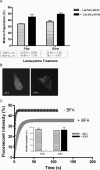
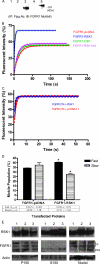
Biochemical and microscopic studies have indicated that FGFR1 is a transmembrane and soluble protein present in the cytosol and nucleus. How FGFR1 enters the cytosol and subsequently the nucleus to control cell development and associated gene activities has become a compelling question. Analyses of protein synthesis, cytoplasmic subcompartmental distribution and movement of FGFR1-EGFP and FGFR1 mutants showed that FGFR1 exists as three separate populations (a) a newly synthesized, highly mobile, nonglycosylated, cytosolic receptor that is depleted by brefeldin A and resides outside the ER-Golgi lumen, (b) a slowly diffusing membrane receptor population, and (c) an immobile membrane pool increased by brefeldin A. RSK1 increases the highly mobile cytosolic FGFR1 population and its overall diffusion rate leading to increased FGFR1 nuclear accumulation, which coaccumulates with RSK1. A model is proposed in which newly synthesized FGFR1 can enter the (a) "nuclear pathway," where the nonglycosylated receptor is extruded from the pre-Golgi producing highly mobile cytosolic receptor molecules that rapidly accumulate in the nucleus or (b) "membrane pathway," in which FGFR1 is processed through the Golgi, where its movement is spatially restricted to trans-Golgi membranes with limited lateral mobility. Entrance into the nuclear pathway is favored by FGFR1's interaction with kinase active RSK1.
Figures


Similar articles
-
Fibroblast growth factor receptor-1 (FGFR1) nuclear dynamics reveal a novel mechanism in transcription control.Mol Biol Cell. 2009 May;20(9):2401-12. doi: 10.1091/mbc.e08-06-0600. Epub 2009 Mar 4. Mol Biol Cell. 2009. PMID: 19261810 Free PMC article.
-
Nuclear trafficking of FGFR1: a role for the transmembrane domain.J Cell Biochem. 2003 Apr 15;88(6):1273-91. doi: 10.1002/jcb.10476. J Cell Biochem. 2003. PMID: 12647309
-
90-kDa ribosomal S6 kinase is a direct target for the nuclear fibroblast growth factor receptor 1 (FGFR1): role in FGFR1 signaling.J Biol Chem. 2004 Jul 9;279(28):29325-35. doi: 10.1074/jbc.M311144200. Epub 2004 Apr 26. J Biol Chem. 2004. PMID: 15117958
-
Integrative nuclear signaling in cell development--a role for FGF receptor-1.DNA Cell Biol. 2007 Dec;26(12):811-26. doi: 10.1089/dna.2007.0664. DNA Cell Biol. 2007. PMID: 18021009 Review.
-
Integrative nuclear FGFR1 signaling (INFS) as a part of a universal "feed-forward-and-gate" signaling module that controls cell growth and differentiation.J Cell Biochem. 2003 Nov 1;90(4):662-91. doi: 10.1002/jcb.10606. J Cell Biochem. 2003. PMID: 14587025 Review.
Cited by
-
Compartmentalization of TNF-related apoptosis-inducing ligand (TRAIL) death receptor functions: emerging role of nuclear TRAIL-R2.Cell Death Dis. 2014 Aug 28;5(8):e1390. doi: 10.1038/cddis.2014.351. Cell Death Dis. 2014. PMID: 25165876 Free PMC article. Review.
-
ProNodal acts via FGFR3 to govern duration of Shh expression in the prechordal mesoderm.Development. 2015 Nov 15;142(22):3821-32. doi: 10.1242/dev.119628. Epub 2015 Sep 28. Development. 2015. PMID: 26417042 Free PMC article.
-
Decoding how receptor tyrosine kinases (RTKs) mediate nuclear calcium signaling.Adv Biol Regul. 2024 May;92:101033. doi: 10.1016/j.jbior.2024.101033. Epub 2024 May 9. Adv Biol Regul. 2024. PMID: 38739986 Free PMC article. Review.
-
NGF-induced cell differentiation and gene activation is mediated by integrative nuclear FGFR1 signaling (INFS).PLoS One. 2013 Jul 10;8(7):e68931. doi: 10.1371/journal.pone.0068931. Print 2013. PLoS One. 2013. PMID: 23874817 Free PMC article.
-
Targeting Cellular Trafficking of Fibroblast Growth Factor Receptors as a Strategy for Selective Cancer Treatment.J Clin Med. 2018 Dec 20;8(1):7. doi: 10.3390/jcm8010007. J Clin Med. 2018. PMID: 30577533 Free PMC article. Review.
References
-
- Bharali, D. J., Klejbor, I., Stachowiak, E. K., Dutta, P., Roy, I., Kaur, N., Bergey, E. J., Prasad, P. N., and Stachowiak, M. K. (2005). Organically modified silica nanoparticles: a nonviral vector for in vivo gene delivery and expression in the brain. Proc. Natl. Acad. Sci. USA 102, 11539–11544. - PMC - PubMed
-
- Carpenter, G. (2003). Nuclear localization and possible functions of receptor tyrosine kinases. Curr. Opin. Cell Biol. 15, 143–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

