Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development
- PMID: 16481421
- PMCID: PMC6674916
- DOI: 10.1523/JNEUROSCI.3202-05.2006
Genetic analyses demonstrate that bone morphogenetic protein signaling is required for embryonic cerebellar development
Abstract
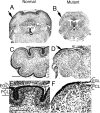
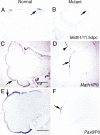
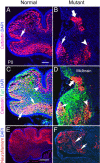
The cerebellum has been a useful model for studying many aspects of neural development because of its relatively simple cytoarchitecture and developmental program. Yet, the genetic mechanisms underlying early differentiation and patterning of the cerebellum are still poorly characterized. Cell expression studies and culture experiments have suggested the importance of bone morphogenetic proteins (BMPs) in development of specific populations of cerebellar neurons. Here, we examined mice with targeted mutations in the BMP type I receptor genes Bmpr1a and Bmpr1b, to genetically test the hypothesis that BMPs play an inductive role in the embryogenesis of cerebellar granule cells. In Bmpr1a;Bmpr1b double knock-out mice, severe cerebellar patterning defects are observed resulting in smaller cerebella that are devoid of foliation. In mutants containing either single BMP receptor gene mutation alone, cerebellar histogenesis appears normal, thereby demonstrating functional redundancy of type I BMP receptors during cerebellar development. Loss of BMP signaling in double mutant animals leads to a dramatic reduction in the number of cerebellar granule cells and ectopic location of many of those that remain. Molecular markers of granule cell specification, including Math1 and Zic1, are drastically downregulated. In addition, Purkinje cells are disorganized and ectopically located, but they appear to be correctly specified. Consistent with the interpretation that granule cells alone are affected, phosphorylated Smad1/5/8 is immunolocalized predominantly to granule cell precursors and not appreciably detected in Purkinje cell precursors. This study demonstrates that BMP signaling plays a crucial role in the specification of granule cells during cerebellar development.
Figures





Similar articles
-
Meis1 Coordinates Cerebellar Granule Cell Development by Regulating Pax6 Transcription, BMP Signaling and Atoh1 Degradation.J Neurosci. 2018 Jan 31;38(5):1277-1294. doi: 10.1523/JNEUROSCI.1545-17.2017. Epub 2018 Jan 9. J Neurosci. 2018. PMID: 29317485 Free PMC article.
-
Common partner Smad-independent canonical bone morphogenetic protein signaling in the specification process of the anterior rhombic lip during cerebellum development.Mol Cell Biol. 2013 May;33(10):1925-37. doi: 10.1128/MCB.01143-12. Epub 2013 Mar 4. Mol Cell Biol. 2013. PMID: 23459943 Free PMC article.
-
Antagonism between Notch and bone morphogenetic protein receptor signaling regulates neurogenesis in the cerebellar rhombic lip.Neural Dev. 2007 Feb 23;2:5. doi: 10.1186/1749-8104-2-5. Neural Dev. 2007. PMID: 17319963 Free PMC article.
-
Bone morphogenetic proteins.Growth Factors. 2004 Dec;22(4):233-41. doi: 10.1080/08977190412331279890. Growth Factors. 2004. PMID: 15621726 Review.
-
BMP/Smad signaling and embryonic cerebellum development: stem cell specification and heterogeneity of anterior rhombic lip.Dev Growth Differ. 2015 Feb;57(2):121-34. doi: 10.1111/dgd.12198. Epub 2015 Feb 23. Dev Growth Differ. 2015. PMID: 25705796 Review.
Cited by
-
Sculptors of cerebellar fissures and their potential as therapeutic targets for cerebellar dysfunction.Front Cell Neurosci. 2025 Jun 5;19:1608185. doi: 10.3389/fncel.2025.1608185. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40538625 Free PMC article. Review.
-
The dynamic role of bone morphogenetic proteins in neural stem cell fate and maturation.Dev Neurobiol. 2012 Jul;72(7):1068-84. doi: 10.1002/dneu.22022. Dev Neurobiol. 2012. PMID: 22489086 Free PMC article. Review.
-
Bone morphogenetic protein-6 promotes cerebellar granule neurons survival by activation of the MEK/ERK/CREB pathway.Mol Biol Cell. 2009 Dec;20(24):5051-63. doi: 10.1091/mbc.e09-05-0424. Mol Biol Cell. 2009. PMID: 19846661 Free PMC article.
-
Development of axon-target specificity of ponto-cerebellar afferents.PLoS Biol. 2011 Feb 8;9(2):e1001013. doi: 10.1371/journal.pbio.1001013. PLoS Biol. 2011. PMID: 21346800 Free PMC article.
-
The transcription factor Zfp423/OAZ is required for cerebellar development and CNS midline patterning.Dev Biol. 2007 Jul 1;307(1):43-52. doi: 10.1016/j.ydbio.2007.04.005. Epub 2007 Apr 12. Dev Biol. 2007. PMID: 17524391 Free PMC article.
References
-
- Ahn K, Mishina Y, Hanks MC, Behringer RR, Crenshaw EB III (2001). BMPR-IA signaling is required for the formation of the apical ectodermal ridge and dorsal-ventral patterning of the limb. Development 128:4449–4461. - PubMed
-
- Akazawa C, Ishibashi M, Shimizu C, Nakanishi S, Kageyama R (1995). A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J Biol Chem 270:8730–8738. - PubMed
-
- Alder J, Cho NK, Hatten ME (1996). Embryonic precursor cells from the rhombic lip are specified to a cerebellar granule neuron identity. Neuron 17:389–399. - PubMed
-
- Alder J, Lee KJ, Jessell TM, Hatten ME (1999). Generation of cerebellar granule neurons in vivo by transplantation of BMP-treated neural progenitor cells. Nat Neurosci 2:535–540. - PubMed
-
- Amy WH, Katherine G, Andrew A, Trisha S, Jane EJ (2001). Overexpression of Math1 disrupts the coordination of neural differentiation in cerebellum development. Mol Cell Neurosci 17:671–682. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
