Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness
- PMID: 16481429
- PMCID: PMC6674945
- DOI: 10.1523/JNEUROSCI.2173-05.2006
Forebrain glucocorticoid receptors modulate anxiety-associated locomotor activation and adrenal responsiveness
Abstract
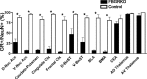
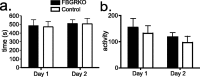
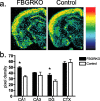
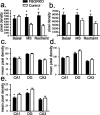
Stress potently modulates anxiety- and depression-related behaviors. In response to stressors, the hypothalamic-pituitary-adrenal (HPA) axis is activated, resulting in the release of glucocorticoids from the adrenal cortex. These hormones act peripherally to restore homeostasis but also feed back to the CNS to control the intensity and duration of the stress response. Glucocorticoids act in limbic areas of the CNS to mediate the psychological and behavioral effects of stress. In this study, we investigate the effect of forebrain-specific disruption of the glucocorticoid receptor (GR) on stress- and anxiety-related behaviors. We demonstrate that mice with disruption of forebrain GR show alterations in stress-induced locomotor activation in a number of anxiety-related behavioral paradigms. These changes are associated with alterations in stress-induced HPA axis activation and, importantly, are not attenuated by chronic treatment with the tricyclic antidepressant imipramine. These data demonstrate the importance of forebrain GR in regulation of physiological and behavioral stress reactivity and suggest that distinct pathways regulate despair- and anxiety-related behaviors.
Figures



References
-
- Barlow DH, Campbell LA (2000). Mixed anxiety-depression and its implications for models of mood and anxiety disorders. Compr Psychiatry 41:55–60. - PubMed
-
- Belzer K, Schneier FR (2004). Comorbidity of anxiety and depressive disorders: issues in conceptualization, assessment, and treatment. J Psychiatr Pract 10:296–306. - PubMed
-
- Bjorntorp P, Rosmond R (2000). The metabolic syndrome—a neuroendocrine disorder? Br J Nutr 83:[Suppl 1], S49–S57. - PubMed
-
- Brewer JA, Khor B, Vogt SK, Muglia LM, Fujiwara H, Haegele KE, Sleckman BP, Muglia LJ (2003). T-cell glucocorticoid receptor is required to suppress COX-2-mediated lethal immune activation. Nat Med 9:1318–1322. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
