Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation
- PMID: 16481433
- PMCID: PMC6674938
- DOI: 10.1523/JNEUROSCI.3918-05.2006
Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation
Abstract
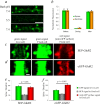
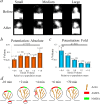
The changes in synaptic morphology and receptor content that underlie neural plasticity are poorly understood. Here, we use a pH-sensitive green fluorescent protein to tag recombinant glutamate receptors and monitor their dynamics onto dendritic spine surfaces. We show that chemically induced long-term potentiation (chemLTP) drives robust exocytosis of AMPA receptors. In contrast, the same stimulus produces a small reduction of NMDA receptors from the spine surface. chemLTP produces similar modification of small and large spines. Interestingly, during chemLTP induction, spines increase in volume before accumulation of AMPA receptors on their surface, indicating that distinct mechanisms underlie changes in morphology and receptor content.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
