Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers
- PMID: 16481441
- PMCID: PMC6674939
- DOI: 10.1523/JNEUROSCI.3574-05.2006
Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers
Abstract
The functional role of heteromers of G-protein-coupled receptors is a matter of debate. In the present study, we demonstrate that heteromerization of adenosine A1 receptors (A1Rs) and A2A receptors (A2ARs) allows adenosine to exert a fine-tuning modulation of glutamatergic neurotransmission. By means of coimmunoprecipitation, bioluminescence and time-resolved fluorescence resonance energy transfer techniques, we showed the existence of A1R-A2AR heteromers in the cell surface of cotransfected cells. Immunogold detection and coimmunoprecipitation experiments indicated that A1R and A2AR are colocalized in the same striatal glutamatergic nerve terminals. Radioligand-binding experiments in cotransfected cells and rat striatum showed that a main biochemical characteristic of the A1R-A2AR heteromer is the ability of A2AR activation to reduce the affinity of the A1R for agonists. This provides a switch mechanism by which low and high concentrations of adenosine inhibit and stimulate, respectively, glutamate release. Furthermore, it is also shown that A1R-A2AR heteromers constitute a unique target for caffeine and that chronic caffeine treatment leads to modifications in the function of the A1R-A2AR heteromer that could underlie the strong tolerance to the psychomotor effects of caffeine.
Figures


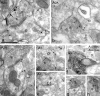



References
-
- Agnati LF, Ferré S, Lluis C, Franco R, Fuxe K (2003). Molecular mechanisms and therapeutical implications of intramembrane receptor/receptor interactions among heptahelical receptors with examples from the striatopallidal GABA neurons. Pharmacol Rev 55:509–550. - PubMed
-
- Andresen BT, Gillespie DG, Mi Z, Dubey RK, Jackson EK (1999). Role of adenosine A(1) receptors in modulating extracellular adenosine levels. J Pharmacol Exp Ther 291:76–80. - PubMed
-
- Antoniou K, Papadopoulou-Daifoti Z, Hyphantis T, Papathanasiou G, Bekris E, Marselos M, Muller M, Goldberg SR, Ferré S (2005). A detailed behavioral analysis on the motor effects of caffeine and the involvement of adenosine A1 and A2A receptors in the rat. Psychopharmacology 183:154–162. - PubMed
-
- Canals M, Marcellino D, Fanelli F, Ciruela F, de Benedetti P, Goldberg SR, Neve K, Fuxe K, Agnati LF, Woods AS, Ferré S, Lluis C, Bouvier M, Franco R (2003). Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J Biol Chem 278:46741–46749. - PubMed
-
- Casadó V, Canti C, Mallol J, Canela EI, Lluis C, Franco R (1990). Solubilization of A1 adenosine receptor from pig brain: characterization and evidence of the role of the cell membrane on the coexistence of high- and low-affinity states. J Neurosci Res 26:461–473. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
