ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury
- PMID: 16481445
- PMCID: PMC6674935
- DOI: 10.1523/JNEUROSCI.4594-05.2006
ErbB2 signaling in Schwann cells is mostly dispensable for maintenance of myelinated peripheral nerves and proliferation of adult Schwann cells after injury
Abstract
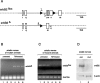
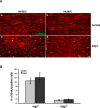
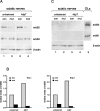
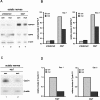
Neuregulin/erbB signaling is critically required for survival and proliferation of Schwann cells as well as for establishing correct myelin thickness of peripheral nerves during development. In this study, we investigated whether erbB2 signaling in Schwann cells is also essential for the maintenance of myelinated peripheral nerves and for Schwann cell proliferation and survival after nerve injury. To this end, we used inducible Cre-loxP technology using a PLP-CreERT2 allele to ablate erbB2 in adult Schwann cells. ErbB2 expression was markedly reduced after induction of erbB2 gene disruption with no apparent effect on the maintenance of already established myelinated peripheral nerves. In contrast to development, Schwann cell proliferation and survival were not impaired in mutant animals after nerve injury, despite reduced levels of MAPK-P (phosphorylated mitogen-activated protein kinase) and cyclin D1. ErbB1 and erbB4 do not compensate for the loss of erbB2. We conclude that adult Schwann cells do not require major neuregulin signaling through erbB2 for proliferation and survival after nerve injury, in contrast to development and in cell culture.
Figures

References
-
- Atanasoski S, Shumas S, Dickson C, Scherer SS, Suter U (2001). Differential cyclin D1 requirements of proliferating Schwann cells during development and after injury. Mol Cell Neurosci 18:581–592. - PubMed
-
- Atanasoski S, Scherer SS, Nave KA, Suter U (2002). Proliferation of Schwann cells and regulation of cyclin D1 expression in an animal model of Charcot-Marie-Tooth disease type 1A. J Neurosci Res 67:443–449. - PubMed
-
- Atanasoski S, Notterpek L, Lee HY, Castagner F, Young P, Ehrengruber MU, Meijer D, Sommer L, Stavnezer E, Colmenares C, Suter U (2004). The protooncogene ski controls Schwann cell proliferation and myelination. Neuron 43:499–511. - PubMed
-
- Atanasoski S, Boller D, De Ventura L, Koegel H, Boentert M, Young P, Werner S, Suter U (2006). Cell cycle inhibitors p21 and p16 are required for the regulation of Schwann cell proliferation. Glia 53:147–157. - PubMed
-
- Awatramani R, Shumas S, Kamholz J, Scherer SS (2002). TGFbeta1 modulates the phenotype of Schwann cells at the transcriptional level. Mol Cell Neurosci 19:307–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
