Distinct roles for cysteine cathepsin genes in multistage tumorigenesis
- PMID: 16481467
- PMCID: PMC1410800
- DOI: 10.1101/gad.1407406
Distinct roles for cysteine cathepsin genes in multistage tumorigenesis
Abstract
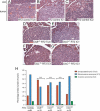
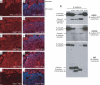
Multiple types of degradative enzymes, including cathepsins of the cysteine protease family, have been implicated in the regulation of angiogenesis and invasion during cancer progression. Several cysteine cathepsins are up-regulated in a mouse model of pancreatic islet cell carcinogenesis (RIP1-Tag2), and tumor progression is impaired following their collective pharmacologic inhibition. Using null mutations of four of the implicated cysteine cathepsins, we have now dissected their individual roles in cancer development. Mutants of cathepsins B or S impaired tumor formation and angiogenesis, while cathepsin B or L knockouts retarded cell proliferation and tumor growth. Absence of any one of these three genes impaired tumor invasion. In contrast, removal of cathepsin C had no effect on either tumor formation or progression. We have identified E-cadherin as a target substrate of cathepsins B, L, and S, but not cathepsin C, potentially explaining their differential effects on tumor invasion. Furthermore, we detected analogous increases in cathepsin expression in human pancreatic endocrine neoplasms, and a significant association between increased levels of cathepsins B and L and tumor malignancy. Thus individual cysteine cathepsin genes make distinctive contributions to tumorigenesis.
Figures






References
-
- Beavon I.R. The E-cadherin–catenin complex in tumour metastasis: Structure, function and regulation. Eur. J. Cancer. 2000;36(13 Spec No):1607–1620. - PubMed
-
- Camp R.L, Charette L.A, Rimm D.L. Validation of tissue microarray technology in breast carcinoma. Lab. Invest. 2000;80:1943–1949. - PubMed
-
- Cavallaro U, Christofori G. Cell adhesion and signalling by cadherins and Ig-CAMs in cancer. Nat. Rev. Cancer. 2004;4:118–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
