A pneumococcal pilus influences virulence and host inflammatory responses
- PMID: 16481624
- PMCID: PMC1368962
- DOI: 10.1073/pnas.0511017103
A pneumococcal pilus influences virulence and host inflammatory responses
Abstract
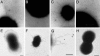
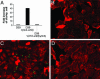
Streptococcus pneumoniae (pneumococcus) is a major cause of morbidity and mortality world-wide. The initial event in invasive pneumococcal disease is the attachment of encapsulated pneumococci to epithelial cells in the upper respiratory tract. This work provides evidence that initial bacterial adhesion and subsequent ability to cause invasive disease is enhanced by pili, long organelles able to extend beyond the polysaccharide capsule, previously unknown to exist in pneumococci. These adhesive pili-like appendages are encoded by the pneumococcal rlrA islet, present in some, but not all, clinical isolates. Introduction of the rlrA islet into an encapsulated rlrA-negative isolate allowed pilus expression, enhanced adherence to lung epithelial cells, and provided a competitive advantage upon mixed intranasal challenge of mice. Furthermore, a pilus-expressing rlrA islet-positive clinical isolate was more virulent than a nonpiliated deletion mutant, and it out-competed the mutant in murine models of colonization, pneumonia, and bacteremia. Additionally, piliated pneumococci evoked a higher TNF response during systemic infection, compared with nonpiliated derivatives, suggesting that pneumococcal pili not only contribute to adherence and virulence but also stimulate the host inflammatory response.
Conflict of interest statement
Conflict of interest statement: M.A.B., M.M., V.M., and R.R. are employees of Chiron Corporation.
Figures

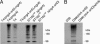



References
-
- Bruyn G. A. W., van Furth R. Eur. J. Clin. Microbiol. Infect. Dis. 1991;10:897–910. - PubMed
-
- Ryan M. W., Antonelli P. J. Laryngoscope. 2000;110:961–964. - PubMed
-
- Cutts F. T., Zaman S. M., Enwere G., Jaffar S., Levine O. S., Okoko C., Oluwalana A., Vaughan S., Obaro A., Leach A., et al. Lancet. 2005;365:1139–1146. - PubMed
-
- Sandgren A., Albiger B., Orihuela C., Tuomanen E., Normark S., Henriques-Normark B. J. Infect. Dis. 2005;192:791–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

