Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus
- PMID: 16483357
- PMCID: PMC1388226
- DOI: 10.1186/1471-2202-7-15
Regulation of glutamatergic signalling by PACAP in the mammalian suprachiasmatic nucleus
Abstract
Background: Previous studies indicate that light information reaches the suprachiasmatic nucleus (SCN) through a subpopulation of retinal ganglion cells that contain both glutamate and pituitary adenylyl cyclase activating peptide (PACAP). While the role of glutamate in this pathway has been well studied, the involvement of PACAP and its receptors are only beginning to be understood. Speculating that PACAP may function to modulate how neurons in the suprachiasmatic nucleus respond to glutamate, we used electrophysiological and calcium imaging tools to examine possible cellular interactions between these co-transmitters.
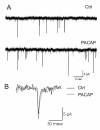
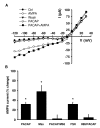
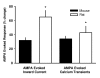
Results: Exogenous application of PACAP increased both the amplitude and frequency of spontaneous excitatory postsynaptic currents recorded from SCN neurons in a mouse brain slice preparation. PACAP also increased the magnitude of AMPA-evoked currents through a mechanism mediated by PAC1 receptors and the adenylyl cyclase-signalling cascade. This enhancement of excitatory currents was not limited to those evoked by AMPA as the magnitude of NMDA currents were also enhanced by application of PACAP. Furthermore, PACAP enhanced AMPA and NMDA evoked calcium transients while PACAP alone produced very little change in resting calcium in most mouse SCN neurons. Finally, in rat SCN neurons, exogenous PACAP enhanced AMPA evoked currents and calcium transients as well evoked robust calcium transients on its own.
Conclusion: The results reported here show that PACAP is a potent modulator of glutamatergic signalling within the SCN in the early night.
Figures


References
-
- Colwell CS, Menaker M. Excitatory Amino Acids: Their Role in Neuroendocrine Function. CRC Press; 1996. Regulation of circadian rhytms by excitatory amino acids; pp. 223–252.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

