Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein
- PMID: 16484190
- PMCID: PMC1426561
- DOI: 10.1128/JB.188.5.1798-1807.2006
Glycosylation of the self-recognizing Escherichia coli Ag43 autotransporter protein
Abstract
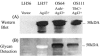
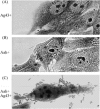
Glycosylation is a common modulation of protein function in eukaryotes and is biologically important. However, in bacteria protein glycosylation is rare, and relatively few bacterial glycoproteins are known. In Escherichia coli only two glycoproteins have been described to date. Here we introduce a novel member to this exclusive group, namely, antigen 43 (Ag43), a self-recognizing autotransporter protein. By mass spectrometry Ag43 was demonstrated to be glycosylated by addition of heptose residues at several positions in the passenger domain. Glycosylation of Ag43 by the action of the Aah and TibC glycosyltransferases was observed in laboratory strains. Importantly, Ag43 was also found to be glycosylated in a wild-type strain, suggesting that Ag43-glycosylation may be a widespread phenomenon. Glycosylation of Ag43 does not seem to interfere with its self-associating properties. However, the glycosylated form of Ag43 enhances bacterial binding to human cell lines, whereas the nonglycosylated version of Ag43 does not to confer this property.
Figures





References
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Bachmann, B. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt, R. Curtis III, J. L. Ingraham, E. C. C. Lin, K. B. Low, B. Magasanik, W. S. Reznikoff, M. Riley, M. Schaechter, and H. E. Umbarger (ed.). Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
-
- Benz, I., and M. A. Schmidt. 2001. Glycosylation with heptose residues mediated by the aah gene product is essential for adherence of the AIDA adhesin. Mol. Microbiol. 40:1403-1413. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

