Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes
- PMID: 16484224
- PMCID: PMC2735442
- DOI: 10.1074/jbc.M511460200
Enhancement of the recycling and activation of beta-adrenergic receptor by Rab4 GTPase in cardiac myocytes
Abstract
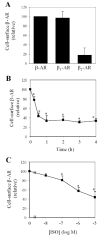
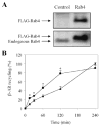
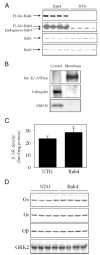
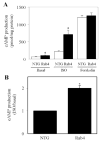
We investigate the role of Rab4, a Ras-like small GTPase coordinating protein transport from the endosome to the plasma membrane, on the recycling and activation of endogenous beta-adrenergic receptor (beta-AR) in HL-1 cardiac myocytes in vitro and transgenic mouse hearts in vivo. Beta1-AR, the predominant subtype of beta-AR in HL-1 cardiac myocytes, was internalized after stimulation with isoproterenol (ISO) and fully recycled at 4 h upon ISO removal. Transient expression of Rab4 markedly facilitated recycling of internalized beta-AR to the cell surface and enhanced beta-AR signaling as measured by ISO-stimulated cAMP production. Transgenic overexpression of Rab4 in the mouse myocardium significantly increased the number of beta-AR in the plasma membrane and augmented cAMP production at the basal level and in response to ISO stimulation. Rab4 overexpression induced concentric cardiac hypertrophy with a moderate increase in ventricle/body weight ratio and posterior wall thickness and a selective up-regulation of the beta-myosin heavy chain gene. These data provide the first evidence indicating that Rab4 is a rate-limiting factor for the recycling of endogenous beta-AR and augmentation of Rab4-mediated traffic enhances beta-AR function in cardiac myocytes.
Figures


Similar articles
-
Regulation of cardiac contractility by Rab4-modulated beta2-adrenergic receptor recycling.Proc Natl Acad Sci U S A. 2004 May 4;101(18):7082-7. doi: 10.1073/pnas.0308335101. Epub 2004 Apr 22. Proc Natl Acad Sci U S A. 2004. PMID: 15105445 Free PMC article.
-
Akt2 deficiency promotes cardiac induction of Rab4a and myocardial β-adrenergic hypersensitivity.J Mol Cell Cardiol. 2010 Dec;49(6):931-40. doi: 10.1016/j.yjmcc.2010.08.011. Epub 2010 Aug 20. J Mol Cell Cardiol. 2010. PMID: 20728450 Free PMC article.
-
cAMP-mediated beta-adrenergic signaling negatively regulates Gq-coupled receptor-mediated fetal gene response in cardiomyocytes.J Mol Cell Cardiol. 2008 Dec;45(6):761-9. doi: 10.1016/j.yjmcc.2008.09.120. Epub 2008 Sep 25. J Mol Cell Cardiol. 2008. PMID: 18851973
-
Regulation of phospholamban and troponin-I phosphorylation in the intact rat cardiomyocytes by adrenergic and cholinergic stimuli: roles of cyclic nucleotides, calcium, protein kinases and phosphatases and depolarization.Mol Cell Biochem. 1995 Aug-Sep;149-150:103-26. doi: 10.1007/BF01076569. Mol Cell Biochem. 1995. PMID: 8569720 Review.
-
Nuclear GPCRs in cardiomyocytes: an insider's view of β-adrenergic receptor signaling.Am J Physiol Heart Circ Physiol. 2011 Nov;301(5):H1754-64. doi: 10.1152/ajpheart.00657.2011. Epub 2011 Sep 2. Am J Physiol Heart Circ Physiol. 2011. PMID: 21890692 Free PMC article. Review.
Cited by
-
CMTM4 regulates angiogenesis by promoting cell surface recycling of VE-cadherin to endothelial adherens junctions.Angiogenesis. 2019 Feb;22(1):75-93. doi: 10.1007/s10456-018-9638-1. Epub 2018 Aug 10. Angiogenesis. 2019. PMID: 30097810 Free PMC article.
-
Internalized Kv1.5 traffics via Rab-dependent pathways.J Physiol. 2008 Oct 15;586(20):4793-813. doi: 10.1113/jphysiol.2008.161570. Epub 2008 Aug 28. J Physiol. 2008. PMID: 18755741 Free PMC article.
-
Role of ubiquitination in endocytic trafficking of G-protein-coupled receptors.Traffic. 2011 Feb;12(2):137-48. doi: 10.1111/j.1600-0854.2010.01121.x. Epub 2010 Oct 15. Traffic. 2011. PMID: 20854416 Free PMC article. Review.
-
RAB43 Promotes Gastric Cancer Cell Proliferation and Metastasis via Regulating the PI3K/AKT Signaling Pathway.Onco Targets Ther. 2020 Mar 11;13:2193-2202. doi: 10.2147/OTT.S237356. eCollection 2020. Onco Targets Ther. 2020. Retraction in: Onco Targets Ther. 2024 Aug 30;17:729-730. doi: 10.2147/OTT.S493691. PMID: 32210585 Free PMC article. Retracted.
-
Rab1 GTPase and dimerization in the cell surface expression of angiotensin II type 2 receptor.J Pharmacol Exp Ther. 2009 Jul;330(1):109-17. doi: 10.1124/jpet.109.153460. Epub 2009 Apr 8. J Pharmacol Exp Ther. 2009. PMID: 19357319 Free PMC article.
References
-
- Xiang Y, Kobilka BK. Science. 2003;300:1530–1532. - PubMed
-
- Post SR, Hammond HK, Insel PA. Annu Rev Pharmacol Toxicol. 1999;39:343–360. - PubMed
-
- Communal C, Singh K, Sawyer DB, Colucci WS. Circulation. 1999;100:2210–2212. - PubMed
-
- Lefkowitz RJ, Rockman HA, Koch WJ. Circulation. 2000;101:1634–1637. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

