Physiological roles of ATP-sensitive K+ channels in smooth muscle
- PMID: 16484295
- PMCID: PMC1779997
- DOI: 10.1113/jphysiol.2006.105973
Physiological roles of ATP-sensitive K+ channels in smooth muscle
Abstract
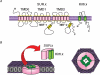
Potassium channels that are inhibited by intracellular ATP (ATP(i)) were first identified in ventricular myocytes, and are referred to as ATP-sensitive K+ channels (i.e. K(ATP) channels). Subsequently, K+ channels with similar characteristics have been demonstrated in many other tissues (pancreatic beta-cells, skeletal muscle, central neurones, smooth muscle). Approximately one decade ago, K(ATP) channels were cloned and were found to be composed of at least two subunits: an inwardly rectifying K+ channel six family (Kir6.x) that forms the ion conducting pore and a modulatory sulphonylurea receptor (SUR) that accounts for several pharmacological properties. Various types of native K(ATP) channels have been identified in a number of visceral and vascular smooth muscles in single-channel recordings. However, little attention has been paid to the molecular properties of the subunits in K(ATP) channels and it is important to determine the relative expression of K(ATP) channel components which give rise to native K(ATP) channels in smooth muscle. The aim of this review is to briefly discuss the current knowledge available for K(ATP) channels with the main interest in the molecular basis of native K(ATP) channels, and to discuss their possible linkage with physiological functions in smooth muscle.
Figures


References
-
- Aguilar-Bryan L, Bryan J. Molecular biology of adenosine triphospate-sensitive potassium channels. Endocrine Rev. 1999;20:101–135. - PubMed
-
- Aschroft FM, Gribble FM. Correlating structure and function in ATP-sensitive K+ channels. Trends Neurosci. 1998;21:288–294. - PubMed
-
- Bonev AD, Nelson MT. Muscarinic inhibition of ATP-sensitive K+ channels by protein kinase C in urinary bladder smooth muscle. Am J Physiol. 1993;265:C1723–C1728. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

