Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis
- PMID: 16484546
- PMCID: PMC2819754
- DOI: 10.1093/jn/136.3.700
Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis
Abstract
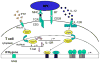
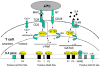
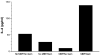
Experimental autoimmune encephalomyelitis (EAE) is a T-cell-mediated, autoimmune disorder characterized by central nervous system inflammation and demyelination, features reminiscent of the human disease, multiple sclerosis (MS). Prior work in the EAE model has suggested that encephalitogenic T cells are of the T helper (Th)-1 phenotype. Our group has performed several studies in the EAE model that suggest that a strategy for treating autoimmune disorders is to convert the pathogenic cells from the Th1 to Th2 phenotype. Peroxisome proliferator-activated receptors (PPARs) are members of a nuclear hormone receptor superfamily that include receptors for steroids, retinoids, and thyroid hormone, all of which are known to affect the immune response. Recently, we examined the role of PPARgamma in EAE and observed that administration of the PPARgamma agonist 15-deoxy-Delta(12,14) prostaglandin J2 exerted a significant therapeutic effect predominantly by inhibiting the activation and expansion of encephalitogenic T cells. One potential advantage in studying PPARalpha agonists is that they have been very well tolerated when used in humans to treat conditions such as elevated triglycerides. Building on prior work in immune deviation and with PPAR agonists, we have demonstrated that PPARalpha agonists can alter the cytokine phenotype of myelin-reactive T cells, alter their encephalitogenicity, and be useful in the treatment of EAE. The fact that PPARalpha agonists have been used as therapeutic agents in humans to treat metabolic conditions for over 25 years with little toxicity makes them attractive candidates for use as adjunctive therapies in MS.
Figures
References
-
- McFarlin DE, McFarland HF. Multiple sclerosis (Part I) N Engl J Med. 1982;307:1183–8. - PubMed
-
- Anderson DW, Ellenberg JH, Leventhal CM, Reingold SC, Rodriguez M, Silberberg DH. Revised estimate of the prevalence of multiple sclerosis in the United States. Ann Neurol. 1992;31:333–6. - PubMed
-
- Martin R, McFarland HF, McFarlin DE. Immunological aspects of demyelinating diseases. Annu Rev Immunol. 1992;10:153–87. - PubMed
-
- Arnason BG. Relevance of experimental allergic encephalomyelitis to multiple sclerosis. Neurol Clin. 1983;1:765–82. - PubMed
-
- IFNB Multiple Sclerosis Study Group. Interferon beta-1b is effective in relapsing-remitting multiple sclerosis I: clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. 1993;43:655–666 [classical article] Neurology. 2001;57:S3–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

