Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness?
- PMID: 16484685
- PMCID: PMC2553364
- DOI: 10.1165/rcmb.2005-0453OC
Do biophysical properties of the airway smooth muscle in culture predict airway hyperresponsiveness?
Abstract
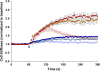
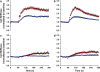
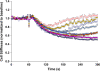
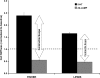
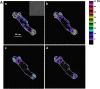
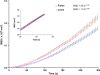
Airway hyperresponsiveness is a cardinal feature of asthma but remains largely unexplained. In asthma, the key end-effector of acute airway narrowing is the airway smooth muscle (ASM) cell. Here we report novel biophysical properties of the ASM cell isolated from the relatively hyporesponsive Lewis rat versus the relatively hyperresponsive Fisher rat. We focused upon the ability of the cytoskeleton (CSK) of the ASM cell to stiffen, to generate contractile forces, and to remodel. We used optical magnetic twisting cytometry to measure cell stiffness and traction microscopy to measure contractile forces. To measure remodeling dynamics, we quantified spontaneous nanoscale motions of a microbead tightly anchored to the CSK. In response to a panel of contractile and relaxing agonists, Fisher ASM cells showed greater stiffening, bigger contractile forces, and faster CSK remodeling; they also exhibited higher effective temperature of the CSK matrix. These physical differences measured at the level of the single cell in vitro were consistent with strain-related differences in airway responsiveness in vivo. As such, comprehensive biophysical characterizations of CSK dynamics at the level of the cell in culture may provide novel perspectives on the ASM and its contributions to the excessive airway narrowing in asthma.
Figures





References
-
- Woolcock AJ, Peat JK. Epidemiology of bronchial hyperresponsiveness. Clin Rev Allergy 1989;7:245–256. - PubMed
-
- Sterk PJ, Bel EH. Bronchial hyperresponsiveness: the need to distinguish between hypersensitivity and excessive airway narrowing. Eur Respir J 1989;2:267–274. - PubMed
-
- Black JL, Johnson PR. Airway smooth muscle in asthma. Respirology 1996;1:153–158. - PubMed
-
- King GG, Pare PD, Seow CY. The mechanics of exaggerated airway narrowing in asthma: the role of smooth muscle. Respir Physiol 1999;118:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

