Why do HIV-1 and HIV-2 use different pathways to develop AZT resistance?
- PMID: 16485036
- PMCID: PMC1364504
- DOI: 10.1371/journal.ppat.0020010
Why do HIV-1 and HIV-2 use different pathways to develop AZT resistance?
Abstract
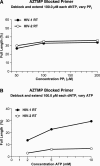
The human immunodeficiency virus type 1 (HIV-1) develops resistance to all available drugs, including the nucleoside analog reverse transcriptase inhibitors (NRTIs) such as AZT. ATP-mediated excision underlies the most common form of HIV-1 resistance to AZT. However, clinical data suggest that when HIV-2 is challenged with AZT, it usually accumulates resistance mutations that cause AZT resistance by reduced incorporation of AZTTP rather than selective excision of AZTMP. We compared the properties of HIV-1 and HIV-2 reverse transcriptase (RT) in vitro. Although both RTs have similar levels of polymerase activity, HIV-1 RT more readily incorporates, and is more susceptible to, inhibition by AZTTP than is HIV-2 RT. Differences in the region around the polymerase active site could explain why HIV-2 RT incorporates AZTTP less efficiently than HIV-1 RT. HIV-1 RT is markedly more efficient at carrying out the excision reaction with ATP as the pyrophosphate donor than is HIV-2 RT. This suggests that HIV-1 RT has a better nascent ATP binding site than HIV-2 RT, making it easier for HIV-1 RT to develop a more effective ATP binding site by mutation. A comparison of HIV-1 and HIV-2 RT shows that there are numerous differences in the putative ATP binding sites that could explain why HIV-1 RT binds ATP more effectively. HIV-1 RT incorporates AZTTP more efficiently than does HIV-2 RT. However, HIV-1 RT is more efficient at ATP-mediated excision of AZTMP than is HIV-2 RT. Mutations in HIV-1 RT conferring AZT resistance tend to increase the efficiency of the ATP-mediated excision pathway, while mutations in HIV-2 RT conferring AZT resistance tend to increase the level of AZTTP exclusion from the polymerase active site. Thus, each RT usually chooses the pathway best suited to extend the properties of the respective wild-type enzymes.
Conflict of interest statement
Figures







References
-
- Sarafianos SG, Das K, Hughes SH, Arnold E. Taking aim at a moving target: Designing drugs to inhibit drug-resistant HIV-1 reverse transcriptases. Curr Opin Struc Biol. 2004;14:716–730. - PubMed
-
- Arion D, Kaushik N, McCormick S, Borkow G, Parniak MA. Phenotypic mechanism of HIV-1 resistance to 3′-azido-3′-deoxythymidine (AZT): Increased polymerization processivity and enhanced sensitivity to pyrophosphate of the mutant viral reverse transcriptase. Biochemistry. 1998;37:15908–15917. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

