Enantioselective disposition of rabeprazole in relation to CYP2C19 genotypes
- PMID: 16487225
- PMCID: PMC1885016
- DOI: 10.1111/j.1365-2125.2005.02566.x
Enantioselective disposition of rabeprazole in relation to CYP2C19 genotypes
Abstract
Aim: Rabeprazole is metabolized to some extent by CYP2C19. The purpose of this study was to elucidate the pharmacokinetics of each rabeprazole enantiomer in three different CYP2C19 genotype groups.
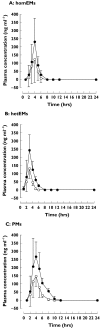
Methods: Twenty-four healthy subjects, of whom each each were homozygous extensive metabolizers (homEMs), heterozygous extensive metabolizers (hetEMs) and poor metabolizers (PMs) for CYP2C19, participated in our study. After a single oral dose of 20 mg of racemic rabeprazole, the plasma concentrations of the rabeprazole enantiomers were measured over the course of 24 h.
Results: The area under the plasma concentration-time curves (AUC) of (R)-rabeprazole in homEMs, hetEMs and PMs were 1.8-, 2.2- and 2.4-fold, respectively, greater than those of (S)-rabeprazole; the relative AUC ratios of (R)- and (S)-rabeprazole in homEMs, hetEMs and PMs were 1:1.1:2.1 and 1:0.9:1.5, respectively. The mean maximum plasma concentrations (Cmax) of (R)-rabeprazole in homEMs, hetEMs and PMs were 1.7-, 1.9- and 1.8-fold higher, respectively, than those of the corresponding (S)-enantiomer (P<0.05). There was no difference between homEMs and PMs in the elimination half-life of (S)-rabeprazole, whereas the elimination half-life of (R)-rabeprazole was significantly longer in PMs than in homEMs [1.7 h (1.4, 2.0) (mean (95% confidence interval)]vs. 0.8 h (0.6, 1.0), respectively, P<0.0001).
Conclusions: (R)-Rabeprazole disposition was influenced to a greater degree by CYP2C19 genetic polymorphisms than was that of (S)-rabeprazole. The effect of CYP2C19 polymorphisms on the stereoselective disposition of rabeprazole was less than those of lansoprazole and omeprazole.
Figures
Similar articles
-
Pharmacokinetic differences between the enantiomers of lansoprazole and its metabolite, 5-hydroxylansoprazole, in relation to CYP2C19 genotypes.Eur J Clin Pharmacol. 2004 Nov;60(9):623-8. doi: 10.1007/s00228-004-0809-1. Epub 2004 Sep 23. Eur J Clin Pharmacol. 2004. PMID: 15448955 Clinical Trial.
-
Enantioselective disposition of lansoprazole in relation to CYP2C19 genotypes in the presence of fluvoxamine.Br J Clin Pharmacol. 2005 Jul;60(1):61-8. doi: 10.1111/j.1365-2125.2005.02381.x. Br J Clin Pharmacol. 2005. PMID: 15963095 Free PMC article. Clinical Trial.
-
Enantioselective disposition of lansoprazole and rabeprazole in human plasma.Yakugaku Zasshi. 2006 Jun;126(6):395-402. doi: 10.1248/yakushi.126.395. Yakugaku Zasshi. 2006. PMID: 16755125 Review.
-
Different effects of fluvoxamine on rabeprazole pharmacokinetics in relation to CYP2C19 genotype status.Br J Clin Pharmacol. 2006 Mar;61(3):309-14. doi: 10.1111/j.1365-2125.2005.02556.x. Br J Clin Pharmacol. 2006. PMID: 16487224 Free PMC article. Clinical Trial.
-
[Recent topics on important drugs for H. pylori eradication: Rabeprazole].Nihon Rinsho. 2005 Nov;63 Suppl 11:350-3. Nihon Rinsho. 2005. PMID: 16363558 Review. Japanese. No abstract available.
Cited by
-
Effect of netazepide, a gastrin/CCK2 receptor antagonist, on gastric acid secretion and rabeprazole-induced hypergastrinaemia in healthy subjects.Br J Clin Pharmacol. 2015 May;79(5):744-55. doi: 10.1111/bcp.12534. Br J Clin Pharmacol. 2015. PMID: 25335860 Free PMC article. Clinical Trial.
-
Efficacy of S-pantoprazole 20 mg compared with pantoprazole 40 mg in the treatment of reflux esophagitis: a randomized, double-blind comparative trial.Dig Dis Sci. 2012 Dec;57(12):3189-94. doi: 10.1007/s10620-012-2297-y. Epub 2012 Jul 8. Dig Dis Sci. 2012. PMID: 22772870 Clinical Trial.
-
Randomized, double-blind, comparative study of dexrabeprazole 10 mg versus rabeprazole 20 mg in the treatment of gastroesophageal reflux disease.World J Gastroenterol. 2007 Aug 14;13(30):4100-2. doi: 10.3748/wjg.v13.i30.4100. World J Gastroenterol. 2007. PMID: 17696229 Free PMC article. Clinical Trial.
-
Stereoselective disposition of proton pump inhibitors.Clin Drug Investig. 2008;28(5):263-79. doi: 10.2165/00044011-200828050-00001. Clin Drug Investig. 2008. PMID: 18407713 Review.
-
A Whole-Body Physiologically Based Pharmacokinetic Model Characterizing Interplay of OCTs and MATEs in Intestine, Liver and Kidney to Predict Drug-Drug Interactions of Metformin with Perpetrators.Pharmaceutics. 2021 May 11;13(5):698. doi: 10.3390/pharmaceutics13050698. Pharmaceutics. 2021. PMID: 34064886 Free PMC article.
References
-
- Prakash A, Faulds D. Rabeprazole. Drugs. 1998;55:261–7. - PubMed
-
- Morii M, Takata H, Fujisaki H, Takeguchi N. The potency of substituted benzimidazoles such as E3810, omeprazole, Ro 18-5364 to inhibit gastric H+, K(+)-ATPase is correlated with the rate of acid-activation of the inhibitor. Biochem Pharmacol. 1990;39:661–7. - PubMed
-
- Fujisaki H, Shibata H, Oketani K, Murakami M, Fujimoto M, Wakabayashi T, Yamatsu I, Yamaguchi M, Sakai H, Takeguchi N. Inhibitions of acid secretion by E3810 and omeprazole, and their reversal by glutathione. Biochem Pharmacol. 1991;42:321–8. - PubMed
-
- Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors — emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13:27–36. - PubMed
-
- Yasuda S, Horai Y, Tomono Y, Nakai H, Yamato C, Manabe K, Kobayashi K, Chiba K, Ishizaki T. Comparison of the kinetic disposition and metabolism of E3810, a new proton pump inhibitor, and omeprazole in relation to S-mephenytoin 4′-hydroxylation status. Clin Pharmacol Ther. 1995;58:143–54. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources