Differential in vitro inhibition of M3G and M6G formation from morphine by (R)- and (S)-methadone and structurally related opioids
- PMID: 16487227
- PMCID: PMC1885024
- DOI: 10.1111/j.1365-2125.2005.02573.x
Differential in vitro inhibition of M3G and M6G formation from morphine by (R)- and (S)-methadone and structurally related opioids
Abstract
Aims: To determine the in vitro kinetics of morphine-3-glucuronide (M3G) and morphine-6-glucuronide (M6G) formation and the inhibition potential by methadone enantiomers and structurally related opioids.
Methods: M3G and M6G formation kinetics from morphine were determined using microsomes from five human livers. Inhibition of glucuronide formation was investigated with eight inhibitors (100 microm) and the mechanism of inhibition determined for (R)- and (S)-methadone (70-500 microm) using three microsomal samples.
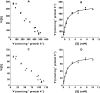
Results: Glucuronide formation displayed single enzyme kinetics. The M3G Vmax (mean+/-SD) was 4.8-fold greater than M6G Vmax (555+/-110 vs. 115+/-19 nmol mg-1 protein h-1; P=0.006, mean of difference 439; 95% confidence interval 313, 565 nmol mg-1 protein h-1). Km values for M3G and M6G formation were not significantly different (1.12+/-0.37 vs. 1.11+/-0.31 mm; P=0.89, 0.02; -0.29, 0.32 mm). M3G and M6G formation was inhibited (P<0.01) with a significant increase in the M3G/M6G ratio (P<0.01) for all compounds tested. Detailed analysis with (R)- and (S)-methadone revealed noncompetitive inhibition with (R)-methadone Ki of 320+/-42 microm and 192+/-12 microm for M3G and M6G, respectively, and (S)-methadone Ki of 226+/-30 microm and 152+/-20 microm for M3G and M6G, respectively. Ki values for M3G inhibition were significantly greater than for M6G for (R)-methadone (P=0.017, 128; 55, 202 microm) and (S)-methadone (P=0.026, 75; 22, 128 microm).
Conclusions: Both methadone enantiomers noncompetitively inhibited the formation of morphine's primary metabolites, with greater inhibition of M6G formation compared with M3G. These findings indicate a mechanism for reduced morphine clearance in methadone-maintained patients and reduced relative formation of the opioid active M6G compared with M3G.
Figures
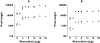

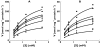
Similar articles
-
The pharmacokinetics of morphine and morphine glucuronide metabolites after subcutaneous bolus injection and subcutaneous infusion of morphine.Br J Clin Pharmacol. 2000 Mar;49(3):207-14. doi: 10.1046/j.1365-2125.2000.00141.x. Br J Clin Pharmacol. 2000. PMID: 10718775 Free PMC article. Clinical Trial.
-
Comparison of the disposition of hepatically-generated morphine-3-glucuronide and morphine-6-glucuronide in isolated perfused liver from the guinea pig.Pharm Res. 1997 Aug;14(8):1014-8. doi: 10.1023/a:1012145126847. Pharm Res. 1997. PMID: 9279882
-
Non-opioid induction of morphine-6-glucuronide synthesis is elicited by prolonged exposure of rat hepatocytes to heroin.Drug Alcohol Depend. 2008 Dec 1;98(3):179-84. doi: 10.1016/j.drugalcdep.2008.05.008. Epub 2008 Jul 1. Drug Alcohol Depend. 2008. PMID: 18597954
-
Morphine metabolites.Acta Anaesthesiol Scand. 1997 Jan;41(1 Pt 2):116-22. doi: 10.1111/j.1399-6576.1997.tb04625.x. Acta Anaesthesiol Scand. 1997. PMID: 9061094 Review.
-
Relationships among morphine metabolism, pain and side effects during long-term treatment: an update.J Pain Symptom Manage. 2003 Jan;25(1):74-91. doi: 10.1016/s0885-3924(02)00531-6. J Pain Symptom Manage. 2003. PMID: 12565191 Review.
Cited by
-
Endogenous opiates and behavior: 2006.Peptides. 2007 Dec;28(12):2435-513. doi: 10.1016/j.peptides.2007.09.002. Epub 2007 Sep 11. Peptides. 2007. PMID: 17949854 Free PMC article. Review.
-
From Pediatric Covariate Model to Semiphysiological Function for Maturation: Part II-Sensitivity to Physiological and Physicochemical Properties.CPT Pharmacometrics Syst Pharmacol. 2012 Oct 10;1(10):e10. doi: 10.1038/psp.2012.12. CPT Pharmacometrics Syst Pharmacol. 2012. PMID: 23887362 Free PMC article.
-
Prediction of morphine clearance in the paediatric population : how accurate are the available pharmacokinetic models?Clin Pharmacokinet. 2012 Nov;51(11):695-709. doi: 10.1007/s40262-012-0006-9. Clin Pharmacokinet. 2012. PMID: 23018467 Review.
-
Pharmacogenetics of Methadone Response.Mol Diagn Ther. 2018 Feb;22(1):57-78. doi: 10.1007/s40291-017-0311-y. Mol Diagn Ther. 2018. PMID: 29168075 Review.
-
Physiologically-Based Pharmacokinetic Model of Morphine and Morphine-3-Glucuronide in Nonalcoholic Steatohepatitis.Clin Pharmacol Ther. 2021 Mar;109(3):676-687. doi: 10.1002/cpt.2037. Epub 2020 Nov 6. Clin Pharmacol Ther. 2021. PMID: 32897538 Free PMC article.
References
-
- Pasternak GW, Bodnar RJ, Clark JA, Inturrisi CE. Morphine-6-glucuronide, a potent mu agonist. Life Sci. 1987;41:2845–9. - PubMed
-
- Klepstad P, Kaasa S, Borchgrevink PC. Start of oral morphine to cancer patients: effective serum morphine concentrations and contribution from morphine-6-glucuronide to the analgesia produced by morphine. Eur J Clin Pharmacol. 2000;55:713–9. - PubMed
-
- Portenoy RK, Thaler HT, Inturrisi CE, Friedlander-Klar H, Foley KM. The metabolite morphine-6-glucuronide contributes to the analgesia produced by morphine infusion in patients with pain and normal renal function. Clin Pharmacol Ther. 1992;51:422–31. - PubMed
-
- Smith GD, Smith MT. Morphine-3-glucuronide: evidence to support its putative role in the development of tolerance to the antinociceptive effects of morphine in the rat. Pain. 1995;62:51–60. - PubMed
-
- Gong QL, Hedner J, Bjorkman R, Hedner T. Morphine-3-glucuronide may functionally antagonize morphine-6-glucuronide induced antinociception and ventilatory depression in the rat. Pain. 1992;48:249–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

