Nickel-induced IL-10 down-regulates Th1- but not Th2-type cytokine responses to the contact allergen nickel
- PMID: 16487249
- PMCID: PMC1809611
- DOI: 10.1111/j.1365-2249.2006.03018.x
Nickel-induced IL-10 down-regulates Th1- but not Th2-type cytokine responses to the contact allergen nickel
Abstract
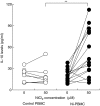
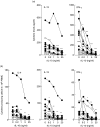
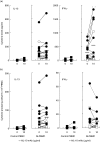
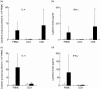
Whereas the involvement of Th1- and Th2-type cytokines in contact allergy to nickel (Ni) is well documented, the role of the regulatory cytokine IL-10 is less clear. We therefore investigated the impact of IL-10 on Ni-induced Th1- (IFN-gamma) and Th2-type (IL-4 and IL-13) cytokine responses in human peripheral blood mononuclear cells (PBMC). PBMC from 15 blood donors with reactivity to Ni (Ni-PBMC) and 8 control donors devoid of reactivity (control PBMC) were stimulated with Ni and the frequency of cytokine-producing cells and the levels of secreted cytokines were analysed by ELISpot (IL-4, IL-13 and IFN-gamma) and ELISA (IL-10, IL-13 and IFN-gamma), respectively. The Ni-induced response was further assessed in the presence of recombinant IL-10 (rIL-10) or neutralizing antibody to IL-10 and the phenotype of the Ni-specific cytokine-producing cells regulated by IL-10 was determined by cell depletion experiments. Ni induced IL-10 production in Ni-PBMC (mean, (range); 33.1 pg/ml (0-93.4 pg/ml)) but not control PBMC (2.2 pg/ml (0-14.9 pg/ml)) (P = 0.002). Ni also induced significant production of IL-4, IL-13 and IFN-gamma that correlated with the IL-10 response. Addition of rIL-10 down-regulated the Ni-induced production of all cytokines but with a more pronounced effect on IFN-gamma. However, neutralization of Ni-induced IL-10 enhanced the levels of IFN-gamma induced by Ni (P = 0.004) but did not affect the number of IFN-gamma-producing cells or the production of other cytokines. Cell depletion experiments suggested that the Ni-specific IFN-gamma (and Th2-type cytokine) producing cells were CD4(+) T cells. The impact of IL-10 on Ni-induced IFN-gamma responses by CD4(+) T cells suggests that an important role of IL-10 in vivo is to counteract the allergic reactions mediated by Th1-type cytokines.
Figures
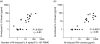
References
-
- Brasch J, Geier J. Patch test results in schoolchildren. Results from the Information Network of Departments of Dermatology (IVDK) and the German Contact Dermatitis Research Group (DKG) Contact Dermatitis. 1997;37:286–93. - PubMed
-
- Schnuch A, Geier J, Uter W, et al. National rates and regional differences in sensitization to allergens of the standard series. Population-adjusted frequencies of sensitization (PAFS) in 40 000 patients from a multicenter study (IVDK) Contact Dermatitis. 1997;37:200–9. - PubMed
-
- Nielsen NH, Linneberg A, Menne T, Madsen F, Frolund L, Dirksen A, Jorgensen T. Incidence of allergic contact sensitization in Danish adults between 1990 and 1998; the Copenhagen Allergy Study, Denmark. Br J Dermatol. 2002;147:487–92. - PubMed
-
- Silvennoinen-Kassinen S, Ikaheimo I, Karvonen J, Kauppinen M, Kallioinen M. Mononuclear cell subsets in the nickel-allergic reaction in vitro and in vivo. J Allergy Clin Immunol. 1992;89:794–800. - PubMed
-
- Shah M, Lewis FM, Gawkrodger DJ. Nickel as an occupational allergen. A survey of 368 nickel-sensitive subjects. Arch Dermatol. 1998;134:1231–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

