Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus
- PMID: 16487251
- PMCID: PMC1809602
- DOI: 10.1111/j.1365-2249.2005.03004.x
Differentiation and immune function of human dendritic cells following infection by respiratory syncytial virus
Abstract
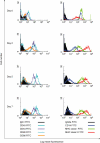
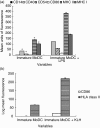
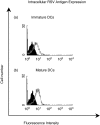
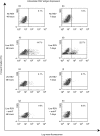
RSV causes annual epidemics of bronchiolitis in winter months resulting in the hospitalization of many infants and the elderly. Dendritic cells (DCs) play a pivotal role in coordinating immune responses to infection and some viruses skew, or subvert, the immune functions of DCs. RSV infection of DCs could alter their function and this could explain why protection after natural RSV infection is incomplete and of short duration. In this study, this interaction between DCs and RSV was investigated using a human primary culture model. DCs were generated from purified healthy adult volunteer peripheral blood monocytes. Effects of RSV upon DC phenotype with RSV primed DCs was measured using flow cytometry. Changes to viability and proliferation of cocultured DCs and T-cells were determined using microscopy with fluorescent dyes (Hoechst 33342 and propidium iodide). DC maturation was not prevented by the RSV challenge. RSV infected a fraction of DCs (10-30%) but the virus replicated slowly in these cells with only small reduction to cell viability. DCs challenged with RSV stimulated T-cell proliferation less well than lipopolysaccharide. This is the first study to demonstrate RSV infection of human monocyte derived DCs and suggests that the virus does not significantly interfere with the function of these cells and potentially may promote cellular rather than humoral responses.
Figures
Similar articles
-
Regulation of mite allergen-pulsed murine dendritic cells by respiratory syncytial virus.Am J Respir Crit Care Med. 2004 Feb 15;169(4):494-8. doi: 10.1164/rccm.200305-663OC. Epub 2003 Dec 4. Am J Respir Crit Care Med. 2004. PMID: 14656751
-
Lung dendritic cells in respiratory syncytial virus bronchiolitis.Pediatr Infect Dis J. 2008 Oct;27(10 Suppl):S89-91. doi: 10.1097/INF.0b013e318168b6f0. Pediatr Infect Dis J. 2008. PMID: 18820586
-
Respiratory syncytial virus differentially activates murine myeloid and plasmacytoid dendritic cells.Immunology. 2007 Sep;122(1):65-72. doi: 10.1111/j.1365-2567.2007.02613.x. Epub 2007 Apr 30. Immunology. 2007. PMID: 17472722 Free PMC article.
-
Immunological mechanisms of severe respiratory syncytial virus bronchiolitis.Intensive Care Med. 2002 May;28(5):616-21. doi: 10.1007/s00134-002-1256-z. Epub 2002 Mar 26. Intensive Care Med. 2002. PMID: 12029411 Review.
-
The role of dendritic cells in innate and adaptive immunity to respiratory syncytial virus, and implications for vaccine development.Expert Rev Vaccines. 2012 Dec;11(12):1441-57. doi: 10.1586/erv.12.117. Expert Rev Vaccines. 2012. PMID: 23252388 Review.
Cited by
-
Dendritic cells in human Pneumovirus and Metapneumovirus infections.Viruses. 2013 Jun 20;5(6):1553-70. doi: 10.3390/v5061553. Viruses. 2013. PMID: 23787776 Free PMC article. Review.
-
Primary human mDC1, mDC2, and pDC dendritic cells are differentially infected and activated by respiratory syncytial virus.PLoS One. 2011 Jan 28;6(1):e16458. doi: 10.1371/journal.pone.0016458. PLoS One. 2011. PMID: 21297989 Free PMC article.
-
The role of the respiratory syncytial virus in airway syndromes in childhood.Curr Allergy Asthma Rep. 2006 Mar;6(2):97-102. doi: 10.1007/s11882-006-0046-z. Curr Allergy Asthma Rep. 2006. PMID: 16566858 Review.
-
Infective respiratory syncytial virus is present in human cord blood samples and most prevalent during winter months.PLoS One. 2017 Apr 24;12(4):e0173738. doi: 10.1371/journal.pone.0173738. eCollection 2017. PLoS One. 2017. PMID: 28437435 Free PMC article.
-
Central role of dendritic cells in shaping the adaptive immune response during respiratory syncytial virus infection.Future Virol. 2011 Aug;6(8):963-973. doi: 10.2217/fvl.11.62. Future Virol. 2011. PMID: 21887154 Free PMC article.
References
-
- Everard ML. Respiratory syncytial virus bronchiolitis and pneumonia. In: Taussig L, Landau L, editors. Textbook of Paediatric Respiratory Medicine. Mosby: St Louis; 1998.
-
- Gilchrist S, Torok TJ, Gary HE, Jr, Alexander JP, Anderson LJ. National surveillance for respiratory syncytial virus, United States 1985–90. J Infectious Dis. 1994;170:986–90. - PubMed
-
- Smith PK, Wang SZ, Dowling KD, Forsyth KD. Leucocyte populations in respiratory syncytial virus-induced bronchiolitis. J Paediatrics Child Health. 2001;37:146–51. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

