Human autoantibodies to diacyl-phosphatidylethanolamine recognize a specific set of discrete cytoplasmic domains
- PMID: 16487257
- PMCID: PMC1809601
- DOI: 10.1111/j.1365-2249.2006.03024.x
Human autoantibodies to diacyl-phosphatidylethanolamine recognize a specific set of discrete cytoplasmic domains
Abstract
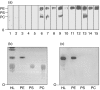
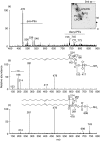
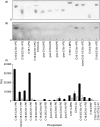
The aim of this study was to characterize a novel human autoantibody-autoantigen system represented as cytoplasmic discrete speckles (CDS) in indirect immunofluorescence (IIF). A distinct CDS IIF pattern represented by 3-20 discrete speckles dispersed throughout the cytoplasm was identified among other cytoplasmic speckled IIF patterns. The cytoplasmic domains labelled by human anti-CDS-1 antibodies did not co-localize with endosome/lysosome markers EEA1 and LAMP-2, but showed partial co-localization with glycine-tryptophan bodies (GWB). CDS-1 sera did not react with several cellular extracts in immunoblotting and did not immunoprecipitate recombinant GW182 or EEA1 proteins. The typical CDS-1 IIF labelling pattern was abolished after delipidation of HEp-2 cells. Moreover, CDS-1 sera reacted strongly with a lipid component co-migrating with phosphatidylethanolamine (PE) in high performance thin-layer chromatography (HPTLC)-immunostaining of HEp-2 cell total lipid extracts. The CDS-1 major molecular targets were established by electrospray ionization-mass spectrometry (ESI-MS), HPTLC-immunostaining and chemiluminescent enzyme-linked immunosorbent assay as diacyl-PE species, containing preferentially a cis-C18 : 1 fatty acid chain at C-2 of the glycerol moiety, namely 1,2-cis-C18 : 1-PE and 1-C16 : 0-2-cis-C18 : 1-PE. The clinical association of CDS-1 sera included a variety of systemic and organ-specific autoimmune diseases but they were also observed in patients with no evidence of autoimmune disease.
Figures



References
-
- Tan EM. Autoantibodies and autoimmunity: a three-decade perspective. A tribute to Henry G. Kunkel. Ann NY Acad Sci. 1997;815:1–14. - PubMed
-
- Humbel RL. Detection of antinuclear antibodies by immunofluorescence. In: van Venrooij WJ, Maini RN, editors. Manual of biological markers of disease. A2. Dordrecht, the Netherlands: Kluwer Academic Publishers; 1993. pp. 1–16.
-
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

