Role of HMGB1 in cardiovascular diseases
- PMID: 16487750
- PMCID: PMC1782046
- DOI: 10.1016/j.coph.2005.10.010
Role of HMGB1 in cardiovascular diseases
Abstract
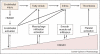
A nuclear protein, high mobility group box 1 (HMGB1), is released passively by necrotic cells, and actively by macrophages/monocytes in response to exogenous and endogenous inflammatory stimuli. After binding to the receptor for advanced glycation end products (RAGE) or toll-like receptor 4 (TLR4), HMGB1 activates vascular endothelial cells and macrophages/monocytes to express proinflammatory cytokines, chemokines and adhesion molecules. Pharmacological suppression of its activities or release is protective against lethal endotoxemia and sepsis, establishing HMGB1 as a critical mediator of lethal systemic inflammation. In light of the pathogenic role of inflammation in cardiovascular diseases, we propose that HMGB1, a proinflammatory cytokine derived from both injured endothelium and activated macrophages/monocytes, could contribute to the progression of atherosclerosis and other cardiovascular diseases.
Figures

References
-
- Wang H, Yang H, Tracey KJ. Extracellular role of HMGB1 in inflammation and sepsis. J Intern Med. 2004;255:320–331. The authors provide a detailed review of the extracellular role of HMGB1 in inflammation as well as lethal systemic inflammatory diseases such as endotoxemia and sepsis. - PubMed
-
- Chen G, Li J, Qiang X, Czura CJ, Ochani M, Ochani K, Ulloa L, Yang H, Tracey KJ, Wang P, et al. Suppression of HMGB1 release by stearoyl lysophosphatidylcholine:an additional mechanism for its therapeutic effects in experimental sepsis. J Lipid Res. 2005;46:623–627. - PubMed
-
- Muller S, Ronfani L, Bianchi ME. Regulated expression and subcellular localization of HMGB1, a chromatin protein with a cytokine function. J Intern Med. 2004;255:332–343. The authors provide an excellent review of the subcellular localization of HMGB1 in various types of cells, and discuss how HMGB1 translocation is tightly regulated at a molecular level. - PubMed
-
- Huttunen HJ, Rauvala H. Amphoterin as an extracellular regulator of cell motility: from discovery to disease. J Intern Med. 2004;255:351–366. In this review, the authors present a detailed review of the role of amphoterin, a cytoplasmic membrane-bound form of HMGB1, in the regulation of cell migration using cell-surface receptors such as RAGE. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

