Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide
- PMID: 16488517
- PMCID: PMC1974903
- DOI: 10.1016/j.vaccine.2006.01.001
Filamentous phage as an immunogenic carrier to elicit focused antibody responses against a synthetic peptide
Abstract
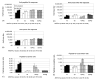
Filamentous bacteriophage are widely used as immunogenic carriers for "phage-displayed" recombinant peptides. Here we report that they are an effective immunogenic carrier for synthetic peptides. The f1.K phage was engineered to have an additional Lys residue near the N-terminus of the major coat protein, pVIII, so as to enhance access to chemical cross-linking agents. The dimeric synthetic peptide, B2.1, was conjugated to f1.K (f1.K/B2.1) in high copy number and compared as an immunogen to B2.1 conjugated to ovalbumin (OVA/B2.1) and to phage-displayed, recombinant B2.1 peptide. All immunogens were administered without adjuvant. The serum antibody titers were measured against: the peptide, the carrier, and, if appropriate, the cross-linker. All immunogens elicited anti-peptide antibody titers, with those elicited by OVA/B2.1 exceeding those by f1.K/B2.1; both titers were greater than that elicited by recombinant B2.1 phage. Comparison of the anti-peptide and anti-carrier antibody responses showed that f1.K/B2.1 elicited a more focused anti-peptide antibody response than OVA/B2.1. The anti-peptide antibody response against f1.K/B2.1 was optimized for the injection route, dose and adjuvant. Dose and adjuvant did not have a significant effect on anti-peptide antibody titers, but a change in injection route from intraperitoneal (IP) to subcutaneous (SC) enhanced anti-peptide antibody titers after seven immunizations. The optimized anti-peptide antibody response exceeded the anti-carrier one by 21-fold, compared to 0.07-fold elicited by OVA/B2.1. This indicates that phage as a carrier can focus the antibody response against the peptide. The results are discussed with respect to the advantages of phage as an alternative to traditional carrier proteins for synthetic peptides, carbohydrates and haptens, and to further improvements in phage as immunogenic carriers.
Figures


References
-
- Grothaus MC, Srivastava N, Smithson SL, Kieber-Emmons T, Williams DB, Carlone GM, et al. Selection of an immunogenic peptide mimic of the capsular polysaccharide of Neisseria meningitidis serogroup A using a peptide display library. Vaccine. 2000;18(13):1253–63. - PubMed
-
- Beenhouwer DO, May RJ, Valadon P, Scharff MD. High affinity mimotope of the polysaccharide capsule of Cryptococcus neoformans identified from an evolutionary phage peptide library. J Immunol. 2002;169(12):6992–9. - PubMed
-
- Pincus SH, Smith MJ, Jennings HJ, Burritt JB, Glee PM. Peptides that mimic the group B streptococcal type III capsular polysaccharide antigen. J Immunol. 1998;160(1):293–8. - PubMed
-
- Prinz DM, Smithson SL, Westerink MA. Two different methods result in the selection of peptides that induce a protective antibody response to Neisseria meningitidis serogroup C. J Immunol Methods. 2004;285(1):1–14. - PubMed
-
- Hou Y, Gu XX. Development of peptide mimotopes of lipooligosaccharide from nontypeable Haemophilus influenzae as vaccine candidates. J Immunol. 2003;170(8):4373–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

