The production and release of living root cap border cells is a function of root apical meristem type in dicotyledonous angiosperm plants
- PMID: 16488922
- PMCID: PMC2803423
- DOI: 10.1093/aob/mcj602
The production and release of living root cap border cells is a function of root apical meristem type in dicotyledonous angiosperm plants
Abstract
Background and aims: The root apical meristems (RAM) of flowering plant roots are organized into recognizable pattern types. At present, there are no known ecological or physiological benefits to having one RAM organization type over another. Although there are phylogenetic distribution patterns in plant groups, the possible evolutionary advantages of different RAM organization patterns are not understood. Root caps of many flowering plant roots are known to release living border cells into the rhizosphere, where the cells are believed to have the capacity to alter conditions in the soil and to interact with soil micro-organisms. Consequently, high rates of border cell production may have the potential to benefit plant growth and development greatly, and to provide a selective advantage in certain soil environments. This study reports the use of several approaches to elucidate the anatomical and developmental relationships between RAM organization and border cell production.
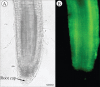
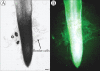
Methods: RAM types from many species were compared with numbers of border cells released in those species. In addition, other species were grown, fixed and sectioned to verify their organization type and capacity to produce border cells. Root tips were examined microscopically to characterize their pattern and some were stained to determine the viability of root cap cells.
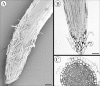
Key results: The first report of a correlation between RAM organization type and the production and release of border cells is provided: species exhibiting open RAM organization produce significantly more border cells than species exhibiting closed apical organization. Roots with closed apical organization release peripheral root cap cells in sheets or large groups of dead cells, whereas root caps with open organization release individual living border cells.
Conclusions: This study, the first to document a relationship between RAM organization, root cap behaviour and a possible ecological benefit to the plant, may yield a framework to examine the evolutionary causes for the diversification of RAM organization types across taxa.
Figures




References
-
- Baum SF, Rost TL. 1996. Root apical organization in Arabidopsis thaliana: 1. Root cap and protoderm. Protoplasma 192: 178–188.
-
- Baum SF, Dubrovsky JG, Rost TL. 2002. Apical organization and maturation events in Arabidopsis thaliana roots: developmental changes over time. American Journal of Botany 89: 908–920. - PubMed
-
- Chapman K, Groot EP, Nichol SA, Rost TL. 2003. Primary root growth and the pattern of root apical meristem organization are coupled. Journal of Plant Growth Regulation 21: 287–295.
-
- Esau K. 1965. Plant anatomy, 2nd edn. New York: John Wiley & Sons, 116–124.
-
- Gladish DK, Rost TL. 1993. The effects of temperature on primary root growth dynamics and lateral root distribution in garden pea (Pisum sativum L., cv. “Alaska”). Environmental and Experimental Botany 33: 1–16.
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

