Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells
- PMID: 16489034
- PMCID: PMC1435866
- DOI: 10.1158/0008-5472.CAN-05-2216
Expression of p53 enhances selenite-induced superoxide production and apoptosis in human prostate cancer cells
Abstract
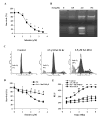
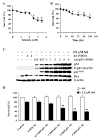
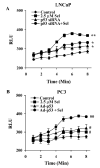
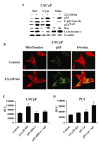
Although the anticancer effects of selenium have been shown in clinical, preclinical, and laboratory studies, the underlying mechanism(s) remains unclear. Our previous study showed that sodium selenite induced LNCaP human prostate cancer cell apoptosis in association with production of reactive oxygen species, alteration of cell redox state, and mitochondrial damage. In the present study, we showed that selenite-induced apoptosis was superoxide mediated and p53 dependent via mitochondrial pathways. In addition, we also showed that superoxide production by selenite was p53 dependent. Our study showed that wild-type p53-expressing LNCaP cells were more sensitive to selenite-induced apoptosis than p53-null PC3 cells. Selenite treatment resulted in high levels of superoxide production in LNCaP cells but only low levels in PC3 cells. LNCaP cells also showed sequential increases in levels of phosphorylated p53 (serine 15), total p53, Bax, and p21(Waf1) proteins following selenite treatment. The effects of selenite were suppressed by pretreatment with a synthetic superoxide dismutase mimic or by knockdown of p53 via RNA interference. LNCaP cells treated with selenite also showed p53 translocation to mitochondria, cytochrome c release into the cytosol, and activation of caspase-9. On the other hand, restoration of wild-type p53 expression in PC3 cells increased cellular sensitivity to selenite and resulted in increased superoxide production, caspase-9 activation, and apoptosis following selenite treatment. These results suggest that selenite induces apoptosis by producing superoxide to activate p53 and to induce p53 mitochondrial translocation. Activation of p53 in turn synergistically enhances superoxide production and apoptosis induced by selenite.
Figures


Similar articles
-
Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells.Cancer Chemother Pharmacol. 2009 Jan;63(2):351-62. doi: 10.1007/s00280-008-0745-3. Epub 2008 Apr 1. Cancer Chemother Pharmacol. 2009. PMID: 18379781 Free PMC article.
-
Inorganic selenium sensitizes prostate cancer cells to TRAIL-induced apoptosis through superoxide/p53/Bax-mediated activation of mitochondrial pathway.Mol Cancer Ther. 2006 Jul;5(7):1873-82. doi: 10.1158/1535-7163.MCT-06-0063. Mol Cancer Ther. 2006. PMID: 16891474
-
Apoptosis induced by selenomethionine and methioninase is superoxide mediated and p53 dependent in human prostate cancer cells.Mol Cancer Ther. 2006 Dec;5(12):3275-84. doi: 10.1158/1535-7163.MCT-06-0400. Mol Cancer Ther. 2006. PMID: 17172431 Free PMC article.
-
Differential involvement of reactive oxygen species in apoptosis induced by two classes of selenium compounds in human prostate cancer cells.Int J Cancer. 2007 May 1;120(9):2034-43. doi: 10.1002/ijc.22480. Int J Cancer. 2007. PMID: 17230520
-
Selenite-induced p53 Ser-15 phosphorylation and caspase-mediated apoptosis in LNCaP human prostate cancer cells.Mol Cancer Ther. 2004 Jul;3(7):877-84. Mol Cancer Ther. 2004. PMID: 15252149
Cited by
-
Sodium selenite induces apoptosis by generation of superoxide via the mitochondrial-dependent pathway in human prostate cancer cells.Cancer Chemother Pharmacol. 2009 Jan;63(2):351-62. doi: 10.1007/s00280-008-0745-3. Epub 2008 Apr 1. Cancer Chemother Pharmacol. 2009. PMID: 18379781 Free PMC article.
-
Health and cellular impacts of air pollutants: from cytoprotection to cytotoxicity.Biochem Res Int. 2012;2012:493894. doi: 10.1155/2012/493894. Epub 2012 Apr 9. Biochem Res Int. 2012. PMID: 22550588 Free PMC article.
-
Selenite as a dual apoptotic and ferroptotic agent synergizes with EGFR and KRAS inhibitors with epigenetic interference.Clin Epigenetics. 2023 Mar 2;15(1):36. doi: 10.1186/s13148-023-01454-4. Clin Epigenetics. 2023. PMID: 36864513 Free PMC article.
-
The effects of selenium on tumor growth in epithelial ovarian carcinoma.J Gynecol Oncol. 2012 Jul;23(3):190-6. doi: 10.3802/jgo.2012.23.3.190. Epub 2012 Jul 2. J Gynecol Oncol. 2012. PMID: 22808362 Free PMC article.
-
High-Dose Selenium Induces Ferroptotic Cell Death in Ovarian Cancer.Int J Mol Sci. 2023 Jan 18;24(3):1918. doi: 10.3390/ijms24031918. Int J Mol Sci. 2023. PMID: 36768241 Free PMC article.
References
-
- Combs GF, Jr, Gray WP. Chemopreventive agents: selenium. Pharmacol Ther. 1998;79:179–92. - PubMed
-
- Ganther HE. Selenium metabolism, selenoproteins and mechanisms of cancer prevention: complexities with thioredoxin reductase. Carcinogenesis. 1999;20:1657–66. - PubMed
-
- Shamberger RJ, Tytko SA, Willis CE. Antioxidants and cancer. Part VI. Selenium and age-adjusted human cancer mortality. Arch Environ Health. 1976;31:231–5. - PubMed
-
- Schrauzer GN, White DA, Schneider CJ. Cancer mortality correlation studies. III. Statistical association with dietary selenium intakes. Bioinorg Chem. 1977;7:35–56. - PubMed
-
- Clark LC, Cantor KP, Allaway WH. Selenium in forage crops and cancer mortality in US counties. Arch Environ Health. 1991;46:37–42. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

