Role of Epstein-Barr virus DNA measurement in plasma in the clinical management of nasopharyngeal carcinoma in a low risk area
- PMID: 16489178
- PMCID: PMC1860284
- DOI: 10.1136/jcp.2005.030544
Role of Epstein-Barr virus DNA measurement in plasma in the clinical management of nasopharyngeal carcinoma in a low risk area
Abstract
Objective: To evaluate the role of quantitative measurement of Epstein-Barr virus (EBV) DNA in the clinical management of nasopharyngeal carcinoma (NPC) in a low tumour risk area (western Europe).
Methods: 22 consecutive Dutch NPC patients (11 europid) were studied. EBV DNA load in pretreatment and post-treatment plasma samples was determined. Three patients were also sampled at frequent intervals during treatment. RNA in situ hybridisation for the detection of EBV encoded RNAs (EBERs) was carried out on tumour biopsies of all cases.
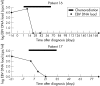
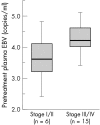
Results: All patients with EBER positive NPC (20/22) showed a positive EBV DNA load in plasma at the time of diagnosis (median EBV DNA level, 4.1 log(10) copies/ml). Patients with EBER negative NPC had no detectable EBV DNA in plasma. After treatment, complete remission was achieved in all cases and concurrently EBV DNA in plasma became undetectable in all patients. In the three longitudinally evaluated cases, EBV DNA load gradually declined towards undetectable levels within three weeks after start of treatment. Two patients developed a distant metastasis with concomitant increases in EBV viral load. In addition, one EBER positive patient developed an EBER negative metastasis in the neck during follow up and in this case EBV DNA load remained undetectable at the time of recurrence.
Conclusions: Plasma EBV DNA load measurement appears to be useful in a low tumour risk area. However, development of local recurrences may not always coincide with raised levels of EBV DNA.
References
-
- Altun M, Fandi A, Dupuis O.et al Undifferentiated nasopharyngeal cancer (UCNT): current diagnostic and therapeutic aspects. Int J Radiat Oncol Biol Phys 199532859–877. - PubMed
-
- McDermott A L, Dutt S N, Watkinson J C. The aetiology of nasopharyngeal carcinoma. Clin Otolaryngol 20012682–92. - PubMed
-
- Fandi A, Cvitkovic E. Biology and treatment of nasopharyngeal cancer. Curr Opin Oncol 19957255–263. - PubMed
-
- Busson P, Keryer C, Ooka T.et al EBV‐associated nasopharyngeal carcinomas: from epidemiology to virus‐targeting strategies. Trends Microbiol 200412356–360. - PubMed
-
- Marks J E, Phillips J L, Menck H R. The National Cancer Database report on the relationship of race and national origin to the histology of nasopharyngeal carcinoma. Cancer 199883582–588. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources