Pilot study of postoperative adjuvant chemoradiation for advanced gastric cancer: adjuvant 5-FU/cisplatin and chemoradiation with capecitabine
- PMID: 16489675
- PMCID: PMC4066094
- DOI: 10.3748/wjg.v12.i4.603
Pilot study of postoperative adjuvant chemoradiation for advanced gastric cancer: adjuvant 5-FU/cisplatin and chemoradiation with capecitabine
Abstract
Aim: To evaluate the efficacy and toxicity of postoperative chemoradiation using FP chemotherapy and oral capecitabine during radiation for advanced gastric cancer following curative resection.
Methods: Thirty-one patients who had underwent a potentially curative resection for Stage III and IV (M0) gastric cancer were enrolled. Therapy consists of one cycle of FP (continuous infusion of 5-FU 1000 mg/m(2) on d 1 to 5 and cisplatin 60 mg/m(2) on d 1) followed by 4500 cGy (180 cGy/d) with capecitabine (1650 mg/m(2) daily throughout radiotherapy). Four wk after completion of the radiotherapy, patients received three additional cycles of FP every three wk. The median follow-up duration was 22.2 mo.
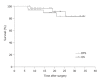
Results: The 3-year disease free and overall survival in this study were 82.7% and 83.4%, respectively. Four patients (12.9%) showed relapse during follow-up. Eight patients did not complete all planned adjuvant therapy. Grade 3/4 toxicities included neutropenia in 50.2%, anemia in 12.9%, thrombocytopenia in 3.2% and nausea/vomiting in 3.2%. Neither grade 3/4 hand foot syndrome nor treatment related febrile neutropenia or death were observed.
Conclusion: These preliminary results suggest that this postoperative adjuvant chemoradiation regimen of FP before and after capecitabine and concurrent radiotherapy appears well tolerated and offers a comparable toxicity profile to the chemoradiation regimen utilized in INT-0116. This treatment modality allowed successful loco-regional control rate and 3-year overall survival.
Figures

Similar articles
-
Adjuvant chemoradiation versus chemotherapy in completely resected advanced gastric cancer with D2 nodal dissection.Asia Pac J Clin Oncol. 2010 Dec;6(4):278-85. doi: 10.1111/j.1743-7563.2010.01331.x. Epub 2010 Oct 26. Asia Pac J Clin Oncol. 2010. PMID: 21114777 Clinical Trial.
-
Phase I study of postoperative radiotherapy combined with capecitabine for gastric cancer.World J Gastroenterol. 2014 Jan 28;20(4):1067-73. doi: 10.3748/wjg.v20.i4.1067. World J Gastroenterol. 2014. PMID: 24574780 Free PMC article. Clinical Trial.
-
Adjuvant bi-weekly combination of cisplatin, infusional 5-fluorouracil and folinic acid followed by concomitant chemoradiotherapy with infusional fluorouracil for high risk operated gastric and gastroesophageal junction adenocarcinoma.Asian Pac J Cancer Prev. 2010;11(6):1493-7. Asian Pac J Cancer Prev. 2010. PMID: 21338186
-
Treatment of localized gastric cancer.Semin Oncol. 2004 Aug;31(4):566-73. doi: 10.1053/j.seminoncol.2004.04.022. Semin Oncol. 2004. PMID: 15297947 Review.
-
Meta-Analysis of Capecitabine versus 5-Fluorouracil in Advanced Gastric Cancer.Evid Based Complement Alternat Med. 2023 Jun 27;2023:4946642. doi: 10.1155/2023/4946642. eCollection 2023. Evid Based Complement Alternat Med. 2023. PMID: 37408581 Free PMC article. Review.
Cited by
-
Postoperative chemoradiation for resected gastric cancer--is the Macdonald Regimen Tolerable? a retrospective multi-institutional study.Radiat Oncol. 2011 Sep 29;6:127. doi: 10.1186/1748-717X-6-127. Radiat Oncol. 2011. PMID: 21958692 Free PMC article.
-
Recent trend in gastric cancer treatment in the USA.J Cancer Metastasis Treat. 2018 Apr 4;4:18. doi: 10.20517/2394-4722.2017.74. Epub 2018 Apr 26. J Cancer Metastasis Treat. 2018. PMID: 34113719 Free PMC article.
-
The recommended treatment strategy for locally advanced gastric cancer in elderly patients aged 75 years and older: a Surveillance, Epidemiology, and End Results database analysis.J Cancer Res Clin Oncol. 2017 Feb;143(2):313-320. doi: 10.1007/s00432-016-2289-y. Epub 2016 Oct 18. J Cancer Res Clin Oncol. 2017. PMID: 27757528 Free PMC article.
-
Four consecutive multicenter phase II trials of adjuvant chemoradiation in patients with completely resected high-risk gastric cancer: the experience of the German AIO/ARO/CAO group.J Cancer Res Clin Oncol. 2009 Feb;135(2):163-72. doi: 10.1007/s00432-008-0463-6. Epub 2008 Sep 30. J Cancer Res Clin Oncol. 2009. PMID: 18825411 Free PMC article.
-
Capecitabine with radiation is an effective adjuvant therapy in gastric cancers.World J Gastroenterol. 2010 Aug 7;16(29):3709-15. doi: 10.3748/wjg.v16.i29.3709. World J Gastroenterol. 2010. PMID: 20677345 Free PMC article.
References
-
- Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, Van de Velde CJ. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993;11:1441–1447. - PubMed
-
- Earle CC, Maroun JA. Adjuvant chemotherapy after curative resection for gastric cancer in non-Asian patients: revisiting a meta-analysis of randomised trials. Eur J Cancer. 1999;35:1059–1064. - PubMed
-
- Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, Haller DG, Ajani JA, Gunderson LL, Jessup JM, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–730. - PubMed
-
- Greene F, Page DL, Fleming ID. Stomach. In: AJCC cancer staging manual., editor. 6th ed. New York: Springer-Verlag Publisher; 2002. pp. 99–106.
-
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, Carbone PP. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–655. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

