The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3
- PMID: 1649007
- PMCID: PMC2711891
- DOI: 10.1016/0092-8674(91)90149-s
The trk tyrosine protein kinase mediates the mitogenic properties of nerve growth factor and neurotrophin-3
Abstract
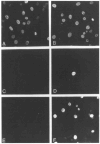
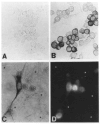
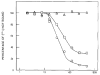
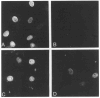
The product of the trk proto-oncogene encodes a receptor for nerve growth factor (NGF). Here we show that NGF is a powerful mitogen that can induce resting NIH 3T3 cells to enter S phase, grow in semisolid medium, and become morphologically transformed. These mitogenic effects are absolutely dependent on expression of gp140trk receptors, but do not require the presence of the previously described low affinity NGF receptor. gp140trk also serves as a receptor for the related factor neurotrophin-3 (NT-3), but not for brain-derived neurotrophic factor. Both NGF and NT-3 induce the rapid phosphorylation of gp140trk receptors and the transient expression of c-Fos proteins. However, NT-3 appears to elicit more limited mitogenic responses than NGF. These results indicate that the product of the trk proto-oncogene is sufficient to mediate signal transduction processes induced by NGF and NT-3, at least in proliferating cells.
Figures




References
-
- Alema S, Casalbore P, Agostini E, Tato F. Differentiation of PC12 phaeochromocytoma cells induced by v-src oncogene. Nature. 1985;316:557–559. - PubMed
-
- Aloe L, Levi-Montalcini R. Mast cells increase in tissues of neonatal rats injected with the nerve growth factor. Brain Res. 1977;133:358–366. - PubMed
-
- Barbacid M, Lamballe F, Pulido D, Klein R. The trk family of tyrosine protein kinase receptors. Biochim Biophys Acta Rev Cancer. 1991 in press. - PubMed
-
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

