Neutrophil elastase causes MUC5AC mucin synthesis via EGF receptor, ERK and NF-kB pathways in A549 cells
- PMID: 16491824
- PMCID: PMC3891072
- DOI: 10.3904/kjim.2005.20.4.275
Neutrophil elastase causes MUC5AC mucin synthesis via EGF receptor, ERK and NF-kB pathways in A549 cells
Abstract
Background: Neutrophil elastase (NE) was found to increase the respiratory mucin gene, MUC5AC, although the molecular mechanisms of this process remain unknown. We attempted to determine the signal transduction pathway through which NE induces MUC5AC gene expression in bronchial epithelial cells.
Methods: A fragment of 1.3 Kb MUC5AC promoter which had been cloned into the pGL3-Basic luciferase vector was transfected to the A549 cells. By measuring the luciferase activity, we were able to evaluate the MUC5AC promoter activity in A549 cells. The involvement of mitogen-activated protein kinases (MAPK) was confirmed by Western blotting. To confirm the involvement of nuclear factorkappaB (NF-kB), we used site-directed mutagenesis and electrophoretic mobility shift assay (EMSA) autoradiogram. The MUC5AC mRNA expression was confirmed by RT-PCR.
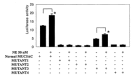
Results: NE increased the transcriptional activity of the MUC5AC promoter in A549 cells. The increased transcriptional activity of the MUC5AC promoter by NE was found to be associated with increased NF-kB activity. Site-directed mutagenesis showed that the transfection of the mutated NF-kB binding sites from the PGL3-MUC5AC-3752 promoter luciferase reporter plasmid decreased the luciferase activity after NE stimulation. Among the MAPKs, only extracellular signal-regulated kinases (ERK) were involved in this NE-induced MUC5AC mucin expression. RT-PCR also showed that NE increased MUC5AC mRNA. An EMSA autoradiogram revealed that NE induced NF-kB:DNA binding.
Conclusions: These results indicate that human NE induces MUC5AC mucin through the epidermal growth factor receptor (EGF-R), ERK, and NF-kB pathways in A549 cells.
Figures





References
-
- Aikawa T, Shimura S, Sasaki H, Ebina M, Takishima T. Marked goblet cell hyperplasia with mucus accumulation in the airways of patients who died of severe acute asthma attack. Chest. 1992;101:916–921. - PubMed
-
- Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J Respir Cell Mol Biol. 1997;17:592–598. - PubMed
-
- Voynow JA, Young LR, Wang Y, Horger T, Rose MC, Fisher BM. Neutrophil elastase increases MUC5AC mRNA and protein expression in respiratory epithelial cells. Am J Physiol. 1999;276:L835–L843. - PubMed
-
- Fischer BM, Voynow JA. Neutrophil elastase induces MUC5AC gene expression in airway epithelium via a pathway involving reactive oxygen species. Am J Respir Cell Mol Biol. 2002;26:447–452. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
