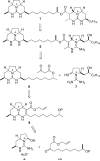
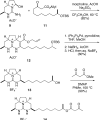
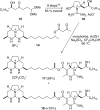
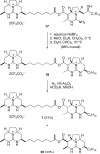
Enantioselective total synthesis of batzelladine F and definition of its structure
- PMID: 16492044
- PMCID: PMC2535800
- DOI: 10.1021/ja057433s
Enantioselective total synthesis of batzelladine F and definition of its structure
Abstract
Batzelladine F (1) was synthesized in enantioselective and stereoselective fashion in 15 steps (longest linear sequence) and 1.7% overall yield from two readily available enantioenriched beta-hydroxy esters, methyl (R)-3-hydroxydecanoate and methyl (R)-3-hydroxybutyrate. Tethered Biginelli condensations are used to assemble both tricyclic guanidine fragments, with the second tethered Biginelli condensation (14 + 16 --> 17) also being employed to join the guanidine fragments. Three diastereomers of batzelladine F, 2-4, were prepared also. A combination of HPLC, optical rotation and CD spectroscopy was employed to distinguish stereoisomers 1-4, proving that 1 is the correct structure of the hexacyclic marine alkaloid batzelladine F.
Figures
Similar articles
-
Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity.Mar Drugs. 2022 Sep 17;20(9):579. doi: 10.3390/md20090579. Mar Drugs. 2022. PMID: 36135769 Free PMC article. Review.
-
Evolution of a strategy for the synthesis of structurally complex batzelladine alkaloids. Enantioselective total synthesis of the proposed structure of batzelladine F and structural revision.J Am Chem Soc. 2006 Mar 1;128(8):2594-603. doi: 10.1021/ja0574320. J Am Chem Soc. 2006. PMID: 16492043 Free PMC article.
-
Enantioselective total synthesis of batzelladine F: structural revision and stereochemical definition.J Am Chem Soc. 2001 Oct 31;123(43):10782-3. doi: 10.1021/ja017067m. J Am Chem Soc. 2001. PMID: 11674029 No abstract available.
-
Application of the Tethered Biginelli Reaction for Enantioselective Synthesis of Batzelladine Alkaloids. Absolute Configuration of the Tricyclic Guanidine Portion of Batzelladine B.J Org Chem. 1999 Mar 5;64(5):1512-1519. doi: 10.1021/jo981971o. J Org Chem. 1999. PMID: 11674213
-
The tethered Biginelli condensation in natural product synthesis.Chem Commun (Camb). 2004 Feb 7;(3):253-65. doi: 10.1039/b309910e. Epub 2003 Oct 27. Chem Commun (Camb). 2004. PMID: 14740029 Review.
Cited by
-
Fused Tricyclic Guanidine Alkaloids: Insights into Their Structure, Synthesis and Bioactivity.Mar Drugs. 2022 Sep 17;20(9):579. doi: 10.3390/md20090579. Mar Drugs. 2022. PMID: 36135769 Free PMC article. Review.
-
Desymmetrization of meso-2,5-diallylpyrrolidinyl ureas through asymmetric palladium-catalyzed carboamination: stereocontrolled synthesis of bicyclic ureas.Angew Chem Int Ed Engl. 2013 Aug 26;52(35):9247-50. doi: 10.1002/anie.201302720. Epub 2013 Jul 3. Angew Chem Int Ed Engl. 2013. PMID: 23824590 Free PMC article.
-
Marine alkaloids as bioactive agents against protozoal neglected tropical diseases and malaria.Nat Prod Rep. 2021 Dec 15;38(12):2214-2235. doi: 10.1039/d0np00078g. Nat Prod Rep. 2021. PMID: 34913053 Free PMC article. Review.
-
A convergent approach to batzelladine alkaloids. Total syntheses of (+)-batzelladine E, (-)-dehydrobatzelladine C, and (+)-batzelladine K.Tetrahedron. 2018 Jun;74(26):3188-3197. doi: 10.1016/j.tet.2018.04.050. Epub 2018 Apr 18. Tetrahedron. 2018. PMID: 33911315 Free PMC article.
-
Syntheses of Cyclic Guanidine-Containing Natural Products.Tetrahedron. 2015 Feb 25;71(8):1145-1173. doi: 10.1016/j.tet.2014.11.056. Tetrahedron. 2015. PMID: 25684829 Free PMC article.
References
-
-
Current Address: Department of Medicinal Chemistry, Genentech, Inc., 1 DNA Way, South San Francisco, CA 94080.
-
-
- Patil AD, Freyer AJ, Taylor PB, Carté B, Zuber G, Johnson RK, Faulkner DJ. J. Org. Chem. 1997;62:1814–1819.
-
-
For reviews summarizing the isolation, structure and synthesis of batzelladine alkaloids, see: Berlinck RGS, Kossuga MH. J. Nat. Prod. 2005;22:516–550. and earlier reviews in this series. For the recent isolation of a new batzelladine alkaloid, see: Gallimore WA, Kelly M, Scheuer PJ. J. Nat. Prod. 2005;68:1420–1423. For a recent synthetic study, see: Arnold MA, Duron SG, Gin DY. J. Am. Chem. Soc. 2005;127:6924–6925.
-
-
- Cohen F, Overman LE. preceding contribution in this issue.
-
-
As discussed in detail in the preceding article, the relative configuration originally proposed for the right-hand tricyclic guanidine fragment of batzelladine F had been revised by the synthesis of model compounds.,
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases