Metallophosphite-catalyzed asymmetric acylation of alpha,beta-unsaturated amides
- PMID: 16492064
- PMCID: PMC1868422
- DOI: 10.1021/ja056018x
Metallophosphite-catalyzed asymmetric acylation of alpha,beta-unsaturated amides
Abstract
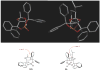
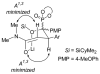
The l-menthone-derived TADDOL phosphite 6b catalyzes highly enantioselective conjugate additions of acyl silanes to alpha,beta-unsaturated amides. p-Methoxybenzoyl cyclohexyldimethylsilane adds to a variety of N,N-dimethyl acrylamide derivatives in the presence of the lithium salt of 6b. In many instances the alpha-silyl-gamma-ketoamide product undergoes facile enantioenrichment (to 97-99% ee) upon recrystallization. Desilylation with HF.pyr affords the formal Stetter addition products. Baeyer-Villiger oxidation of the desilylated gamma-ketoamides affords useful ester products. An X-ray diffraction study of 6b reveals that the isopropyl group of the menthone ketal influences the position of the syn-pseudoaxial phenyl group in the TADDOL structure. Through a crossover experiment, the silicon migration step in the reaction mechanism is shown to be strictly intramolecular.
Figures
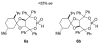

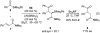
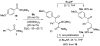


Similar articles
-
Enantioselective metallophosphite-catalyzed C-acylation of nitrones.J Am Chem Soc. 2007 Oct 31;129(43):12944-5. doi: 10.1021/ja076095n. Epub 2007 Oct 10. J Am Chem Soc. 2007. PMID: 17927189 Free PMC article. No abstract available.
-
TADDOL-derived phosphites and phosphoramidites for efficient rhodium-catalyzed asymmetric hydroboration.Org Lett. 2006 Jul 6;8(14):3097-100. doi: 10.1021/ol061117g. Org Lett. 2006. PMID: 16805561
-
Highly enantioselective Pictet-Spengler reactions with alpha-ketoamide-derived ketimines: access to an unusual class of quaternary alpha-amino amides.Angew Chem Int Ed Engl. 2009;48(13):2403-6. doi: 10.1002/anie.200806110. Angew Chem Int Ed Engl. 2009. PMID: 19222080
-
Remarkable levels of enantioswitching in catalytic asymmetric hydroboration.Org Lett. 2010 Oct 15;12(20):4612-5. doi: 10.1021/ol101932q. Org Lett. 2010. PMID: 20863120 Free PMC article.
-
Enantioselective total syntheses of several bioactive natural products based on the development of practical asymmetric catalysis.Chem Pharm Bull (Tokyo). 2004 Sep;52(9):1031-52. doi: 10.1248/cpb.52.1031. Chem Pharm Bull (Tokyo). 2004. PMID: 15340187 Review.
Cited by
-
Synthesis of Complex Glycolates by Enantioconvergent Addition Reactions.Acc Chem Res. 2017 Sep 19;50(9):2284-2296. doi: 10.1021/acs.accounts.7b00263. Epub 2017 Aug 17. Acc Chem Res. 2017. PMID: 28817258 Free PMC article.
-
The Catalytic Asymmetric Intramolecular Stetter Reaction.Synlett. 2009 May;8(2009):1189-1207. doi: 10.1055/s-0029-1216654. Synlett. 2009. PMID: 20585467 Free PMC article.
-
Towards Tartaric-Acid-Derived Asymmetric Organocatalysts.European J Org Chem. 2013 Jul;2013(21):4471-4482. doi: 10.1002/ejoc.201201675. Epub 2013 Jun 3. European J Org Chem. 2013. PMID: 24194674 Free PMC article.
-
Enantio- and diastereoselective intermolecular Stetter reaction of glyoxamide and alkylidene ketoamides.Org Lett. 2009 Jul 2;11(13):2856-9. doi: 10.1021/ol901081a. Org Lett. 2009. PMID: 19507841 Free PMC article.
-
Enantioselective metallophosphite-catalyzed C-acylation of nitrones.J Am Chem Soc. 2007 Oct 31;129(43):12944-5. doi: 10.1021/ja076095n. Epub 2007 Oct 10. J Am Chem Soc. 2007. PMID: 17927189 Free PMC article. No abstract available.
References
-
- Stetter H, Kuhlmann H. Org React (N Y) 1991;40:407–496.
-
- Seebach D. Angew Chem, Int Ed Engl. 1979;18:239–258.
-
- Johnson JS. Angew Chem, Int Ed. 2004;43:1326–1328. - PubMed
-
-
For an exception using PBu3, see Gong JH, Im YJ, Lee KY, Kim JN. Tetrahedron Lett. 2002;43:1247–1251.
-
-
- Enders D, Breuer K, Runsink J, Teles JH. Helv Chim Acta. 1996;79:1899–1902.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

