Metallophosphite-catalyzed asymmetric acylation of alpha,beta-unsaturated amides
- PMID: 16492064
- PMCID: PMC1868422
- DOI: 10.1021/ja056018x
Metallophosphite-catalyzed asymmetric acylation of alpha,beta-unsaturated amides
Abstract
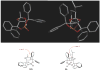
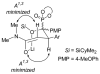
The l-menthone-derived TADDOL phosphite 6b catalyzes highly enantioselective conjugate additions of acyl silanes to alpha,beta-unsaturated amides. p-Methoxybenzoyl cyclohexyldimethylsilane adds to a variety of N,N-dimethyl acrylamide derivatives in the presence of the lithium salt of 6b. In many instances the alpha-silyl-gamma-ketoamide product undergoes facile enantioenrichment (to 97-99% ee) upon recrystallization. Desilylation with HF.pyr affords the formal Stetter addition products. Baeyer-Villiger oxidation of the desilylated gamma-ketoamides affords useful ester products. An X-ray diffraction study of 6b reveals that the isopropyl group of the menthone ketal influences the position of the syn-pseudoaxial phenyl group in the TADDOL structure. Through a crossover experiment, the silicon migration step in the reaction mechanism is shown to be strictly intramolecular.
Figures
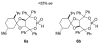

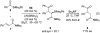
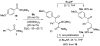


References
-
- Stetter H, Kuhlmann H. Org React (N Y) 1991;40:407–496.
-
- Seebach D. Angew Chem, Int Ed Engl. 1979;18:239–258.
-
- Johnson JS. Angew Chem, Int Ed. 2004;43:1326–1328. - PubMed
-
-
For an exception using PBu3, see Gong JH, Im YJ, Lee KY, Kim JN. Tetrahedron Lett. 2002;43:1247–1251.
-
-
- Enders D, Breuer K, Runsink J, Teles JH. Helv Chim Acta. 1996;79:1899–1902.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

