Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis
- PMID: 16492732
- PMCID: PMC1413927
- DOI: 10.1073/pnas.0511290103
Prion protein (PrPc) positively regulates neural precursor proliferation during developmental and adult mammalian neurogenesis
Abstract
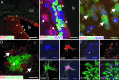
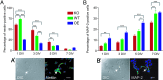
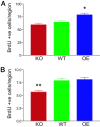
The misfolding of the prion protein (PrP(c)) is a central event in prion diseases, yet the normal function of PrP(c) remains unknown. PrP(c) has putative roles in many cellular processes including signaling, survival, adhesion, and differentiation. Given the abundance of PrP(c) in the developing and mature mammalian CNS, we investigated the role of PrP(c) in neural development and in adult neurogenesis, which occurs constitutively in the dentate gyrus (DG) of the hippocampus and in the olfactory bulb from precursors in the subventricular zone (SVZ)/rostral migratory stream. In vivo, we find that PrP(c) is expressed immediately adjacent to the proliferative region of the SVZ but not in mitotic cells. In vivo and in vitro studies further find that PrP(c) is expressed in multipotent neural precursors and mature neurons but is not detectable in glia. Loss- and gain-of-function experiments demonstrate that PrP(c) levels correlate with differentiation of multipotent neural precursors into mature neurons in vitro and that PrP(c) levels positively influence neuronal differentiation in a dose-dependent manner. PrP(c) also increases cellular proliferation in vivo; in the SVZ, PrP(c) overexpresser (OE) mice have more proliferating cells compared with wild-type (WT) or knockout (KO) mice; in the DG, PrP(c) OE and WT mice have more proliferating cells compared with KO mice. Our results demonstrate that PrP(c) plays an important role in neurogenesis and differentiation. Because the final number of neurons produced in the DG is unchanged by PrP(c) expression, other factors must control the ultimate fate of new neurons.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Aguzzi A., Polymenidou M. Cell. 2004;116:313–327. - PubMed
-
- Hetz C., Maundrell K., Soto C. Trends Mol. Med. 2003;9:237–243. - PubMed
-
- Bueler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Cell. 1993;73:1339–1347. - PubMed
-
- Kanaani J., Prusiner S. B., Diacovo J., Baekkeskov S., Legname G. J. Neurochem. 2005;95:1373–1386. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

