IL-32, a proinflammatory cytokine in rheumatoid arthritis
- PMID: 16492735
- PMCID: PMC1413916
- DOI: 10.1073/pnas.0511233103
IL-32, a proinflammatory cytokine in rheumatoid arthritis
Abstract
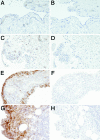
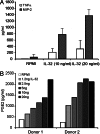
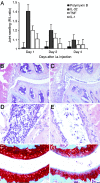
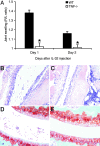
IL-32 is a recently discovered cytokine that induces TNFalpha, IL-1beta, IL-6, and chemokines. We investigated whether IL-32 is expressed in the synovia of patients with rheumatoid arthritis (RA) and studied associations with disease severity and the presence of other cytokines. Immunohistochemistry revealed that IL-32 is highly expressed in RA synovial tissue biopsies, whereas IL-32 was not observed in synovial tissues from patients with osteoarthritis. Moreover, in synovial biopsies from 29 RA patients with active disease, the level of IL-32 staining correlated with erythrocyte sedimentation rate, a marker of systemic inflammation (R = 0.63 and P < 0.0003). Synovial staining of IL-32 also correlated with indices of synovial inflammation (R = 0.80 and P < 0.0001) as well as synovial presence of TNFalpha (R = 0.68 and P < 0.004), IL-1beta (R = 0.79 and P < 0.0001), and IL-18 (R = 0.82 and P < 0.001). IL-32 was a potent inducer of prostaglandin E(2) release in mouse macrophages and human blood monocytes, an important property for inflammation. After the injection of human IL-32gamma into the knee joints of naïve mice, joint swelling, with pronounced influx of inflammatory cells and cartilage damage, was observed. In TNFalpha-deficient mice, IL-32-driven joint swelling was absent and cell influx was markedly reduced, but loss of proteoglycan was unaffected, suggesting that IL-32 activity is, in part, TNFalpha-dependent. IL-32, strongly associated with TNFalpha, IL-1beta, and IL-18, appears to play a role in human RA and may be a novel target in autoimmune diseases.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Arend W. P., Dayer J.-M. Arthritis Rheum. 1995;38:151–160. - PubMed
-
- Feldmann M., Brennan F. M., Maini R. N. Annu. Rev. Immunol. 1996;14:397–440. - PubMed
-
- Miossec P., van den Berg W. Arthritis Rheum. 1997;40:2105–2115. - PubMed
-
- Bresnihan B., Tak P. P. Baillieres Best. Pract. Res. Clin. Rheumatol. 1999;13:645–659. - PubMed
-
- Goronzy J. J., Weyand C. M. Immunol. Rev. 2005;204:55–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

