Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3
- PMID: 16492736
- PMCID: PMC1413914
- DOI: 10.1073/pnas.0511207103
Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3
Abstract
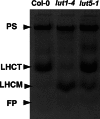
Lutein, a dihydroxy derivative of alpha-carotene (beta,epsilon-carotene), is the most abundant carotenoid in photosynthetic plant tissues where it plays important roles in light-harvesting complex-II structure and function. The synthesis of lutein from lycopene requires at least four distinct enzymatic reactions: beta- and epsilon-ring cyclizations and hydroxylation of each ring at the C-3 position. Three carotenoid hydroxylases have already been identified in Arabidopsis, two nonheme diiron beta-ring monooxygenases (the B1 and B2 loci) that primarily catalyze hydroxylation of the beta-ring of beta,beta-carotenoids and one heme-containing monooxygenase (CYP97C1, the LUT1 locus) that catalyzes hydroxylation of the epsilon-ring of beta,epsilon-carotenoids. In this study, we demonstrate that Arabidopsis CYP97A3 (the LUT5 locus) encodes a fourth carotenoid hydroxylase with major in vivo activity toward the beta-ring of alpha-carotene (beta,epsilon-carotene) and minor activity on the beta-rings of beta-carotene (beta,beta-carotene). A cyp97a3-null allele, lut5-1, causes an accumulation of alpha-carotene at a level equivalent to beta-carotene in wild type, which is stably incorporated into photosystems, and a 35% reduction in beta-carotene-derived xanthophylls. That lut5-1 still produces 80% of wild-type lutein levels, indicating at least one of the other carotene hydroxylases, can partially compensate for the loss of CYP97A3 activity. From these data, we propose a model for the preferred pathway for lutein synthesis in plants: ring cyclizations to form alpha-carotene, beta-ring hydroxylation of alpha-carotene by CYP97A3 to produce zeinoxanthin, followed by epsilon-ring hydroxylation of zeinoxanthin by CYP97C1 to produce lutein.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

 , ε-cyclization;
, ε-cyclization;  , β-cyclization;
, β-cyclization;  , β-ring hydroxylation of β,ε- and β,ψ-carotenoids;
, β-ring hydroxylation of β,ε- and β,ψ-carotenoids;  , ε-ring hydroxylation;,
, ε-ring hydroxylation;,  , β-ring hydroxylation of β,β-carotenoids. Reactions blocked by mutation of the indicated loci are shown: lut1 (ε-ring hydroxylase), lut2 (ε-cyclase), lut5, b1, and b2 (three β-ring hydroxylases). Solid gray arrows indicate a reaction sequence that is supported by mutant phenotypes and/or enzyme activity assays in E. coli, whereas dashed gray arrows are not. Solid black arrows, compounds, and mutant loci indicate major biosynthetic routes.
, β-ring hydroxylation of β,β-carotenoids. Reactions blocked by mutation of the indicated loci are shown: lut1 (ε-ring hydroxylase), lut2 (ε-cyclase), lut5, b1, and b2 (three β-ring hydroxylases). Solid gray arrows indicate a reaction sequence that is supported by mutant phenotypes and/or enzyme activity assays in E. coli, whereas dashed gray arrows are not. Solid black arrows, compounds, and mutant loci indicate major biosynthetic routes.

Similar articles
-
The Arabidopsis LUT1 locus encodes a member of the cytochrome p450 family that is required for carotenoid epsilon-ring hydroxylation activity.Proc Natl Acad Sci U S A. 2004 Jan 6;101(1):402-7. doi: 10.1073/pnas.2237237100. Proc Natl Acad Sci U S A. 2004. PMID: 14709673 Free PMC article.
-
Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis.Plant Cell. 2003 Jun;15(6):1320-32. doi: 10.1105/tpc.011403. Plant Cell. 2003. PMID: 12782726 Free PMC article.
-
Over-expression of Arabidopsis thaliana carotenoid hydroxylases individually and in combination with a beta-carotene ketolase provides insight into in vivo functions.Phytochemistry. 2010 Feb;71(2-3):168-78. doi: 10.1016/j.phytochem.2009.10.011. Epub 2009 Nov 24. Phytochemistry. 2010. PMID: 19939422
-
Progress in understanding the origin and functions of carotenoid hydroxylases in plants.Arch Biochem Biophys. 2004 Oct 1;430(1):22-9. doi: 10.1016/j.abb.2004.02.003. Arch Biochem Biophys. 2004. PMID: 15325908 Review.
-
Carotenoid hydroxylation--P450 finally!Trends Plant Sci. 2004 Nov;9(11):515-7. doi: 10.1016/j.tplants.2004.09.001. Trends Plant Sci. 2004. PMID: 15501174 Review.
Cited by
-
Systematic identification and characterization of genes in the regulation and biogenesis of photosynthetic machinery.Cell. 2023 Dec 7;186(25):5638-5655.e25. doi: 10.1016/j.cell.2023.11.007. Cell. 2023. PMID: 38065083 Free PMC article.
-
Comparative transcriptional profiling analysis of developing melon (Cucumis melo L.) fruit from climacteric and non-climacteric varieties.BMC Genomics. 2015 Jun 9;16(1):440. doi: 10.1186/s12864-015-1649-3. BMC Genomics. 2015. PMID: 26054931 Free PMC article.
-
KIPEs3: Automatic annotation of biosynthesis pathways.PLoS One. 2023 Nov 16;18(11):e0294342. doi: 10.1371/journal.pone.0294342. eCollection 2023. PLoS One. 2023. PMID: 37972102 Free PMC article.
-
Color components determination and full-length comparative transcriptomic analyses reveal the potential mechanism of carotenoid synthesis during Paphiopedilum armeniacum flowering.PeerJ. 2024 Feb 22;12:e16914. doi: 10.7717/peerj.16914. eCollection 2024. PeerJ. 2024. PMID: 38406281 Free PMC article.
-
Carotenoid Accumulation and Its Contribution to Flower Coloration of Osmanthus fragrans.Front Plant Sci. 2018 Oct 16;9:1499. doi: 10.3389/fpls.2018.01499. eCollection 2018. Front Plant Sci. 2018. PMID: 30459779 Free PMC article.
References
-
- Straub O. In: Key to Carotenoids. Pfander H., Gerspacher M., Rychener M., Schwabe R., editors. Basel: Birkhäuser; 1987. pp. 11–218.
-
- Kull D., Pfander H. In: Carotenoids. Britton G., Liaaen-Jensen S., Pfander H., editors. Basel: Birkhäuser; 1995. pp. 295–316.
-
- Johnson E. A., Schroeder W. A. Adv. Biochem. Eng. Biotechnol. 1996;53:119–178. - PubMed
-
- Liaaen-Jensen S. In: Carotenoids: Biosynthesis and Metabolism. Britton G., Liaaen-Jensen S., Pfander H., editors. Vol. 3. Basel: Birkhäuser; 1998. pp. 217–247.
-
- Yoshii Y., Hanyuda T., Wakana I., Miyaji K., Arai S., Ueda K., Inouye I. J. Phycol. 2004;40:1170–1177.
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

