Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease
- PMID: 16492752
- PMCID: PMC1413946
- DOI: 10.1073/pnas.0600134103
Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer's disease
Abstract
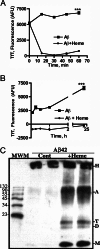
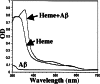
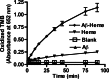
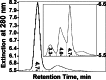
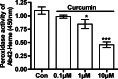
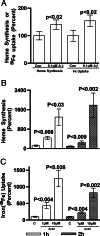
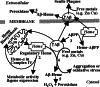
Amyloid-beta peptide (Abeta) is the toxic agent in Alzheimer's disease (AD), although the mechanism causing the neurodegeneration is not known. We previously proposed a mechanism in which excessive Abeta binds to regulatory heme, triggering functional heme deficiency (HD), causing the key cytopathologies of AD. We demonstrated that HD triggers the release of oxidants (e.g., H(2)O(2)) from mitochondria due to the loss of complex IV, which contains heme-a. Now we add more evidence that Abeta binding to regulatory heme in vivo is the mechanism by which Abeta causes HD. Heme binds to Abeta, thus preventing Abeta aggregation by forming an Abeta-heme complex in a cell-free system. We suggest that this complex depletes regulatory heme, which would explain the increase in heme synthesis and iron uptake we observe in human neuroblastoma cells. The Abeta-heme complex is shown to be a peroxidase, which catalyzes the oxidation of serotonin and 3,4-dihydroxyphenylalanine by H(2)O(2). Curcumin, which lowers oxidative damage in the brain in a mouse model for AD, inhibits this peroxidase. The binding of Abeta to heme supports a unifying mechanism by which excessive Abeta induces HD, causes oxidative damage to macromolecules, and depletes specific neurotransmitters. The relevance of the binding of regulatory heme with excessive Abeta for mitochondrial dysfunction and neurotoxicity and other cytopathologies of AD is discussed.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
References
-
- Billings L. M., Oddo S., Green K. N., McGaugh J. L., Laferla F. M. Neuron. 2005;45:675–688. - PubMed
-
- Walsh D. M., Selkoe D. J. Protein Pept. Lett. 2004;11:213–228. - PubMed
-
- Smith M. A., Nunomura A., Zhu X., Takeda A., Perry G. Antioxid. Redox. Signal. 2000;2:413–420. - PubMed
-
- Parker W. D., Jr, Parks J., Filley C. M., Kleinschmidt-DeMasters B. K. Neurology. 1994;44:1090–1096. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

