Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation
- PMID: 16492756
- PMCID: PMC1413933
- DOI: 10.1073/pnas.0511315103
Monovalent ligation of the B cell receptor induces receptor activation but fails to promote antigen presentation
Abstract
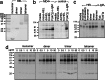
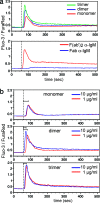
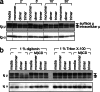
We explored the role of antigen valency in B cell receptor (BCR) activation and rearrangement of intracellular MHC class II compartments as factors that contribute to the efficacy of antigen presentation. Using primary B cells that express a hen egg lysozyme (HEL)-specific BCR, we found that oligomeric HEL more efficiently promoted both BCR activation and internalization than did monovalent HEL, although monovalent HEL, unlike monovalent Fab fragments of anti-Ig, readily triggered the BCR. Nonetheless, oligovalent ligation positions the BCR in a membrane microdomain that is distinct from one engaged in the course of monovalent ligation, as judged by detergent extraction of the BCR. Furthermore, oligovalent HEL induced more pronounced rearrangement of MHC class II-containing antigen-processing compartments. Using videomicroscopy we observed in real time the rearrangement of MHC class II compartments as well as delivery of antigen in primary B cells. The observed increase in rearrangement of MHC class II-positive compartments and the disposition of antigen-bound BCRs therein correlates with improved presentation of a HEL-derived epitope. Although monomeric HEL efficiently engages the BCR, presentation of HEL-derived epitopes is impaired compared to oligovalent antigens. This trait may help explain the known ability of soluble, disaggregated antigen to induce a state of B cell tolerance.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
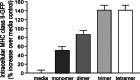

Similar articles
-
Efficiency of antigen presentation after antigen targeting to surface IgD, IgM, MHC, Fc gamma RII, and B220 molecules on murine splenic B cells.J Immunol. 1989 Jul 1;143(1):59-65. J Immunol. 1989. PMID: 2471743
-
Efficient presentation of multivalent antigens targeted to various cell surface molecules of dendritic cells and surface Ig of antigen-specific B cells.J Immunol. 1998 Dec 1;161(11):6059-67. J Immunol. 1998. PMID: 9834089
-
Simultaneous presentation and cross-presentation of immune-stimulating complex-associated cognate antigen by antigen-specific B cells.Eur J Immunol. 2008 May;38(5):1238-46. doi: 10.1002/eji.200737758. Eur J Immunol. 2008. PMID: 18398931
-
Viewing the antigen-induced initiation of B-cell activation in living cells.Immunol Rev. 2008 Feb;221:64-76. doi: 10.1111/j.1600-065X.2008.00583.x. Immunol Rev. 2008. PMID: 18275475 Review.
-
Antigen presentation by B lymphocytes: how receptor signaling directs membrane trafficking.Curr Opin Immunol. 2007 Feb;19(1):93-8. doi: 10.1016/j.coi.2006.11.011. Epub 2006 Nov 30. Curr Opin Immunol. 2007. PMID: 17140785 Review.
Cited by
-
Valency and density matter: Deciphering impacts of immunogen structures on immune responses against a tumor associated carbohydrate antigen using synthetic glycopolymers.Biomaterials. 2016 Sep;101:189-98. doi: 10.1016/j.biomaterials.2016.05.050. Epub 2016 Jun 2. Biomaterials. 2016. PMID: 27294537 Free PMC article.
-
Antigen presentation and the ubiquitin-proteasome system in host-pathogen interactions.Adv Immunol. 2006;92:225-305. doi: 10.1016/S0065-2776(06)92006-9. Adv Immunol. 2006. PMID: 17145306 Free PMC article. Review.
-
TG2-gluten complexes as antigens for gluten-specific and transglutaminase-2 specific B cells in celiac disease.PLoS One. 2021 Nov 3;16(11):e0259082. doi: 10.1371/journal.pone.0259082. eCollection 2021. PLoS One. 2021. PMID: 34731200 Free PMC article.
-
Dynamics and activation of membrane-bound B cell receptor assembly.Commun Biol. 2025 Feb 13;8(1):226. doi: 10.1038/s42003-025-07478-1. Commun Biol. 2025. PMID: 39948415 Free PMC article.
-
Monte Carlo study of B-cell receptor clustering mediated by antigen crosslinking and directed transport.Cell Mol Immunol. 2011 May;8(3):255-64. doi: 10.1038/cmi.2011.3. Epub 2011 Feb 28. Cell Mol Immunol. 2011. PMID: 21358668 Free PMC article.
References
-
- Dal Porto J. M., Gauld S. B., Merrell K. T., Mills D., Pugh-Bernard A. E., Cambier J. Mol. Immunol. 2004;41:599–613. - PubMed
-
- Campbell K. S. Curr. Opin. Immunol. 1999;11:256–264. - PubMed
-
- Lanzavecchia A. Annu. Rev. Immunol. 1990;8:773–793. - PubMed
-
- Parker D. C. Annu. Rev. Immunol. 1993;11:331–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

