Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex
- PMID: 16492764
- PMCID: PMC1413920
- DOI: 10.1073/pnas.0511264103
Homodimeric cross-over structure of the human granulocyte colony-stimulating factor (GCSF) receptor signaling complex
Abstract
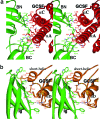
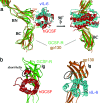
A crystal structure of the signaling complex between human granulocyte colony-stimulating factor (GCSF) and a ligand binding region of GCSF receptor (GCSF-R), has been determined to 2.8 A resolution. The GCSF:GCSF-R complex formed a 2:2 stoichiometry by means of a cross-over interaction between the Ig-like domains of GCSF-R and GCSF. The conformation of the complex is quite different from that between human GCSF and the cytokine receptor homologous domain of mouse GCSF-R, but similar to that of the IL-6/gp130 signaling complex. The Ig-like domain cross-over structure necessary for GCSF-R activation is consistent with previously reported thermodynamic and mutational analyses.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


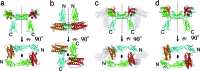
References
-
- Nagata S., Tsuchiya M., Asano S., Kaziro Y., Yamazaki T., Yamamoto O., Hirata Y., Kubota N., Oheda M., Nomura H., et al. Nature. 1986;319:415–418. - PubMed
-
- Metcalf D. Nature. 1990;339:27–30. - PubMed
-
- Welte K., Gabrilove J., Bronchud M. H., Platzer E., Morstyn G. Blood. 1996;88:1907–1929. - PubMed
-
- Fukunaga R., Ishizaka-Ikeda E., Seto Y., Nagata S. Cell. 1990;61:341–350. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

