Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes
- PMID: 16492770
- PMCID: PMC1413908
- DOI: 10.1073/pnas.0511062103
Proteomic analysis of adaptor protein 1A coats selectively assembled on liposomes
Abstract
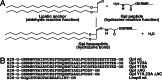
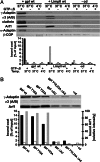
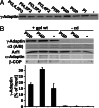
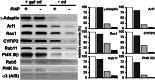
Coat components localize to specific membrane domains, where they sort selected transmembrane proteins. To study how clathrin coats are stabilized on such domains and to identify the protein networks involved, we combined proteomic screens and in vitro liposome-based assays that recapitulate the fidelity of protein sorting in vivo. Our study identifying approximately 40 proteins on AP-1A-coated liposomes revealed that AP-1A coat assembly triggers the concomitant recruitment of Rac1, its effectors, and the Wave/Scar complex as well as that of Rab11 and Rab14. The coordinated recruitment of these different machineries requires a mosaic of membrane components comprising the GTPase ADP-ribosylation factor 1, sorting signals in selected transmembrane proteins, and phosphatidylinositol 4-phosphate. These results demonstrate that the combinatorial use of low-affinity binding sites present on the same membrane domain accounts not only for a selective coat assembly but also for the coordinated assembly of selected machineries required for actin polymerization and subsequent membrane fusion.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

References
-
- Kirchhausen T. Nat. Rev. Mol. Cell Biol. 2000;1:187–198. - PubMed
-
- Antonny B., Schekman R. Curr. Opin. Cell Biol. 2001;13:438–443. - PubMed
-
- Bonifacino J. S., Traub L. M. Annu. Rev. Biochem. 2003;72:395–447. - PubMed
-
- Owen D. J., Collins B. M., Evans P. R. Annu. Rev. Cell Dev. Biol. 2004;20:153–191. - PubMed
-
- Robinson M. S. Trends Cell Biol. 2004;14:167–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

