High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize
- PMID: 16492777
- PMCID: PMC1413895
- DOI: 10.1073/pnas.0509650103
High frequency of centromere inactivation resulting in stable dicentric chromosomes of maize
Abstract
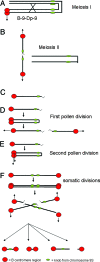
Somatic chromosome spreads from maize (Zea mays L.) plants containing B-A translocation chromosomes undergoing the chromosome type breakage-fusion-bridge cycle were examined by FISH. The size and type of extra chromosomes varied among cells of the same individual. A collection of minichromosomes derived from the chromosome type breakage-fusion-bridge cycle was examined for the presence of stable dicentric chromosomes. Six of 23 chromosomes in the collection contained two regions with DNA sequences typical of centromeres. Functional analysis and immunolabeling of CENH3, the centromere-specific histone H3 variant, revealed only one functional centromere per chromosome, despite the duplicate centromere sequences. One plant was found with an inactive B centromere that had been translocated to the short arm of chromosome 9. The translocated centromere region appeared identical to that of a normal B chromosome. The inactivation of the centromeres was stable for at least four generations. By using dicentrics from dispensable chromosomes, centromere inactivation was found to be quite common under these circumstances.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures
Similar articles
-
Sequential de novo centromere formation and inactivation on a chromosomal fragment in maize.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1263-71. doi: 10.1073/pnas.1418248112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733907 Free PMC article.
-
Dicentric chromosome formation and epigenetics of centromere formation in plants.J Genet Genomics. 2012 Mar 20;39(3):125-30. doi: 10.1016/j.jgg.2012.01.006. Epub 2012 Feb 14. J Genet Genomics. 2012. PMID: 22464471 Review.
-
Sequences associated with A chromosome centromeres are present throughout the maize B chromosome.Chromosoma. 2005 Feb;113(7):337-49. doi: 10.1007/s00412-004-0319-z. Epub 2004 Dec 7. Chromosoma. 2005. PMID: 15586285
-
Rapid Birth or Death of Centromeres on Fragmented Chromosomes in Maize.Plant Cell. 2020 Oct;32(10):3113-3123. doi: 10.1105/tpc.20.00389. Epub 2020 Aug 18. Plant Cell. 2020. PMID: 32817254 Free PMC article.
-
Maize centromeres: structure, function, epigenetics.Annu Rev Genet. 2009;43:287-303. doi: 10.1146/annurev-genet-102108-134834. Annu Rev Genet. 2009. PMID: 19689211 Review.
Cited by
-
Sequential de novo centromere formation and inactivation on a chromosomal fragment in maize.Proc Natl Acad Sci U S A. 2015 Mar 17;112(11):E1263-71. doi: 10.1073/pnas.1418248112. Epub 2015 Mar 2. Proc Natl Acad Sci U S A. 2015. PMID: 25733907 Free PMC article.
-
Cytogenetic identification and molecular marker development of a novel wheat-Leymus mollis 4Ns(4D) alien disomic substitution line with resistance to stripe rust and Fusarium head blight.Front Plant Sci. 2022 Nov 1;13:1012939. doi: 10.3389/fpls.2022.1012939. eCollection 2022. Front Plant Sci. 2022. PMID: 36407596 Free PMC article.
-
Cytological analysis of the diploid-like inheritance of newly synthesized allotetraploid wheat.Chromosome Res. 2023 Dec 18;32(1):1. doi: 10.1007/s10577-023-09745-5. Chromosome Res. 2023. PMID: 38108925
-
Rice as a model for centromere and heterochromatin research.Chromosome Res. 2007;15(1):77-84. doi: 10.1007/s10577-006-1104-z. Chromosome Res. 2007. PMID: 17295128 Review.
-
The Physical Location of Stripe Rust Resistance Genes on Chromosome 6 of Rye (Secale cereale L.) AR106BONE.Front Plant Sci. 2022 Jun 29;13:928014. doi: 10.3389/fpls.2022.928014. eCollection 2022. Front Plant Sci. 2022. PMID: 35845635 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

