The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest
- PMID: 16492778
- PMCID: PMC1413893
- DOI: 10.1073/pnas.0509417103
The Vif and Vpr accessory proteins independently cause HIV-1-induced T cell cytopathicity and cell cycle arrest
Abstract
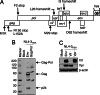
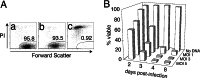
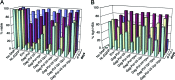
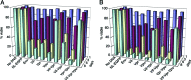
HIV type I (HIV-1) can cause G(2) cell cycle arrest and death of CD4(+) T lymphocytes in vitro and inexorable depletion of these cells in vivo. However, the molecular mechanism of viral cytopathicity has not been satisfactorily elucidated. Previously, we showed that HIV-1 kills T cells by a necrotic form of cell death that requires high level expression of an integrated provirus but not the env or nef genes. To determine which viral protein(s) are required for cell death, we systematically mutated, alone and in combination, the ORFs of the NL4-3 strain of HIV-1. We found that the elimination of the viral functions encoded by gag-pol and vpu, tat, and rev did not mitigate cytopathicity. However, elimination of the vif and vpr accessory genes together, but not individually, renders the virus incapable of causing cell death and G(2) cell cycle blockade. We thus identify vif and vpr as necessary for T cell cytopathic effects induced by HIV-1. These findings may provide an important insight into the molecular mechanism of viral pathogenesis in AIDS.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures

Similar articles
-
The Vif accessory protein alters the cell cycle of human immunodeficiency virus type 1 infected cells.Virology. 2007 Mar 15;359(2):243-52. doi: 10.1016/j.virol.2006.09.026. Epub 2006 Oct 23. Virology. 2007. PMID: 17056089 Free PMC article.
-
gag, vif, and nef genes contribute to the homologous viral interference induced by a nonproducer human immunodeficiency virus type 1 (HIV-1) variant: identification of novel HIV-1-inhibiting viral protein mutants.J Virol. 1998 May;72(5):4308-19. doi: 10.1128/JVI.72.5.4308-4319.1998. J Virol. 1998. PMID: 9557721 Free PMC article.
-
Role of viral regulatory and accessory proteins in HIV-1 replication.Front Biosci. 2004 Sep 1;9:2388-413. doi: 10.2741/1403. Front Biosci. 2004. PMID: 15353294 Review.
-
Expression of human immunodeficiency virus type 1 vif and vpr mRNAs is Rev-dependent and regulated by splicing.Virology. 1991 Aug;183(2):677-86. doi: 10.1016/0042-6822(91)90996-o. Virology. 1991. PMID: 1830183
-
The HIV-1 Vpu protein: a multifunctional enhancer of viral particle release.Microbes Infect. 2003 Sep;5(11):1029-39. doi: 10.1016/s1286-4579(03)00191-6. Microbes Infect. 2003. PMID: 12941395 Review.
Cited by
-
From reactivation of latent HIV-1 to elimination of the latent reservoir: the presence of multiple barriers to viral eradication.Bioessays. 2013 Jun;35(6):544-52. doi: 10.1002/bies.201200170. Epub 2013 Apr 24. Bioessays. 2013. PMID: 23613347 Free PMC article. Review.
-
Clearance of HIV-1 or SIV reservoirs by promotion of apoptosis and inhibition of autophagy: Targeting intracellular molecules in cure-directed strategies.J Leukoc Biol. 2022 Nov;112(5):1245-1259. doi: 10.1002/JLB.4MR0222-606. Epub 2022 Mar 31. J Leukoc Biol. 2022. PMID: 35362118 Free PMC article. Review.
-
β-caryophyllene oxide induces apoptosis and inhibits proliferation of A549 lung cancer cells.Med Oncol. 2023 May 26;40(7):189. doi: 10.1007/s12032-023-02022-9. Med Oncol. 2023. PMID: 37233859 Free PMC article.
-
The Impact of HIV- and ART-Induced Mitochondrial Dysfunction in Cellular Senescence and Aging.Cells. 2021 Jan 16;10(1):174. doi: 10.3390/cells10010174. Cells. 2021. PMID: 33467074 Free PMC article. Review.
-
The role of cullin 5-containing ubiquitin ligases.Cell Div. 2016 Mar 9;11:1. doi: 10.1186/s13008-016-0016-3. eCollection 2016. Cell Div. 2016. PMID: 27030794 Free PMC article. Review.
References
-
- Gallo R. C. Immunol. Rev. 2002;185:236–265. - PubMed
-
- Ho D. D., Neumann A. U., Perelson A. S., Chen W., Leonard J. M., Markowitz M. Nature. 1995;373:123–126. - PubMed
-
- Perelson A. S., Neumann A. U., Markowitz M., Leonard J. M., Ho D. D. Science. 1996;271:1582–1586. - PubMed
-
- Wei X., Ghosh S. K., Taylor M. E., Johnson V. A., Emini E. A., Deutsch P., Lifson J. D., Bonhoeffer S., Nowak M. A., Hahn B. H., et al. Nature. 1995;373:117–122. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

