Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration
- PMID: 16492779
- PMCID: PMC1413892
- DOI: 10.1073/pnas.0509397103
Cyclic GMP-independent mechanisms contribute to the inhibition of platelet adhesion by nitric oxide donor: a role for alpha-actinin nitration
Abstract
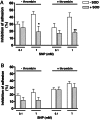
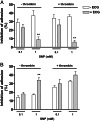
The nitric oxide-mediated actions are mostly due to cyclic GMP (cGMP) formation, but cGMP-independent mechanisms, such as tyrosine nitration, have been suggested as potential signaling pathways modulating the NO-induced responses. However, the mechanisms that lead to tyrosine nitration in platelets are poorly studied, and the protein targets of nitration have not been identified in these cells. Therefore, we have used the model of platelet adhesion to fibrinogen-coated plates to investigate the cGMP-independent mechanisms of the NO-donor sodium nitroprusside (SNP) that leads to inhibition of platelet adhesion. SNP concentration-dependently inhibited platelet adhesion, as observed at 15-min and 60-min adhesion. Additionally, SNP markedly increased the cGMP levels, and the soluble guanylate inhibitor ODQ nearly abolished the SNP-mediated cGMP elevations in all experimental conditions used. Nevertheless, ODQ failed to affect the adhesion inhibition obtained with 1.0 mM SNP at 15 min. On the other hand, superoxide dismutase or peroxynitrite (ONOO(-)) scavenger epigallocatechin gallate significantly reversed the inhibition of platelet adhesion by SNP (1 mM, 15 min). Western blot analysis in SNP (1 mM, 15 min)-treated platelets showed a single tyrosine-nitrated protein with an apparent mass of approximately 105 kDa. Nanospray LC-MS/MS identified the human alpha-actinin 1 cytoskeletal isoform (P12814) as the protein contained in the nitrated SDS gel band. Thus, tyrosine nitration of alpha-actinin, through ONOO(-) formation, may be a key modulatory mechanism to control platelet adhesion.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures




Similar articles
-
The Importance of Alpha-Actinin Proteins in Platelet Formation and Function, and Their Causative Role in Congenital Macrothrombocytopenia.Int J Mol Sci. 2021 Aug 29;22(17):9363. doi: 10.3390/ijms22179363. Int J Mol Sci. 2021. PMID: 34502272 Free PMC article. Review.
-
The role of superoxide anion in the inhibitory effect of SIN-1 in thrombin-activated human platelet adhesion.Eur J Pharmacol. 2010 Feb 10;627(1-3):229-34. doi: 10.1016/j.ejphar.2009.10.060. Epub 2009 Nov 4. Eur J Pharmacol. 2010. PMID: 19895807
-
Nitric oxide inhibits platelet adhesion to collagen through cGMP-dependent and independent mechanisms: the potential role for S-nitrosylation.Platelets. 2009 Nov;20(7):478-86. doi: 10.3109/09537100903159375. Platelets. 2009. PMID: 19852686
-
Activation of haem-oxidized soluble guanylyl cyclase with BAY 60-2770 in human platelets lead to overstimulation of the cyclic GMP signaling pathway.PLoS One. 2012;7(11):e47223. doi: 10.1371/journal.pone.0047223. Epub 2012 Nov 8. PLoS One. 2012. PMID: 23144808 Free PMC article.
-
Evidence for, and importance of, cGMP-independent mechanisms with NO and NO donors on blood vessels and platelets.Curr Vasc Pharmacol. 2005 Jan;3(1):41-53. doi: 10.2174/1570161052773933. Curr Vasc Pharmacol. 2005. PMID: 15638781 Review.
Cited by
-
The Importance of Alpha-Actinin Proteins in Platelet Formation and Function, and Their Causative Role in Congenital Macrothrombocytopenia.Int J Mol Sci. 2021 Aug 29;22(17):9363. doi: 10.3390/ijms22179363. Int J Mol Sci. 2021. PMID: 34502272 Free PMC article. Review.
-
Clotting Dysfunction in Sepsis: A Role for ROS and Potential for Therapeutic Intervention.Antioxidants (Basel). 2021 Dec 30;11(1):88. doi: 10.3390/antiox11010088. Antioxidants (Basel). 2021. PMID: 35052592 Free PMC article. Review.
-
Lack of effect of ODQ does not exclude cGMP signalling via NO-sensitive guanylyl cyclase.Br J Pharmacol. 2013 Sep;170(2):317-27. doi: 10.1111/bph.12275. Br J Pharmacol. 2013. PMID: 23763290 Free PMC article.
-
cGMP modulation therapeutics for sickle cell disease.Exp Biol Med (Maywood). 2019 Feb;244(2):132-146. doi: 10.1177/1535370219827276. Epub 2019 Jan 28. Exp Biol Med (Maywood). 2019. PMID: 30691292 Free PMC article. Review.
-
Antiplatelet effects of dietary nitrate in healthy volunteers: involvement of cGMP and influence of sex.Free Radic Biol Med. 2013 Dec;65:1521-1532. doi: 10.1016/j.freeradbiomed.2013.06.031. Epub 2013 Jun 24. Free Radic Biol Med. 2013. PMID: 23806384 Free PMC article. Clinical Trial.
References
-
- Dopheide S. M., Yap C. L., Jackson S. P. Clin. Exp. Pharmacol. Physiol. 2001;28:355–363. - PubMed
-
- Niiya K., Hodson E., Bader R., Byers-Ward V., Koziol J. A., Plow E. F., Ruggeri Z. M. Blood. 1987;70:475–483. - PubMed
-
- Savage B., Shattil S. J., Ruggeri Z. M. J. Biol. Chem. 1992;267:11300–11306. - PubMed
-
- Nolte C., Eigentaler M., Horstrup K., Honig-Liedl P., Walter U. Biochem. Pharmacol. 1994;48:1569–1575. - PubMed
-
- Noack E., Feelisch M. Basic Res. Cardiol. 1991;2:37–50. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

