Cooperativity in the self-assembly of porphyrin ladders
- PMID: 16492786
- PMCID: PMC1413879
- DOI: 10.1073/pnas.0508071103
Cooperativity in the self-assembly of porphyrin ladders
Abstract
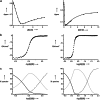
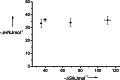
Cooperativity is a general feature of intermolecular interactions in biomolecular systems, but there are many different facets of the phenomenon that are not well understood. Positive cooperativity stabilizes a system as progressively more interactions are added, and the origin of the beneficial free energy may be entropic or enthalpic in origin. An "enthalpic chelate effect" has been proposed to operate through structural tightening that improves all of the functional group interactions in a complex, when it is more strongly bound. Here, we present direct calorimetric evidence that no such enthalpic effects exist in the cooperative assembly of supramolecular ladder complexes composed of metalloporphyrin oligomers coordinated to bipyridine ligands. The enthalpic contributions of the individual coordination interactions are 35 kJ.mol(-1) and constant over a range of free energies of self-assembly of -35 to -111 kJ.mol(-1). In rigid well defined systems of this type, the enthalpies of individual interactions are additive, and no enthalpic cooperative effects are apparent. The implication is that in more flexible, less well defined systems such as biomolecular assemblies, the enthalpy contributions available from specific functional group interactions are well defined and constant parameters.
Conflict of interest statement
Conflict of interest statement: No conflicts declared.
Figures


Similar articles
-
Cooperativity, partially bound states, and enthalpy-entropy compensation.Chem Biol. 2003 Nov;10(11):1023-32. doi: 10.1016/j.chembiol.2003.10.009. Chem Biol. 2003. PMID: 14652069
-
Enthalpic and entropic components of cooperativity for the partially ligated intermediates of hemoglobin support a "symmetry rule" mechanism.Biochemistry. 1995 May 16;34(19):6316-27. doi: 10.1021/bi00019a009. Biochemistry. 1995. PMID: 7756259
-
Water accessibility to the binding cleft as a major switching factor from entropy-driven to enthalpy-driven binding of an alkyl group by synthetic receptors.Chem Asian J. 2010 May 3;5(5):1163-70. doi: 10.1002/asia.200900679. Chem Asian J. 2010. PMID: 20379991
-
Energetic and entropic factors determining binding affinity in protein-ligand complexes.J Recept Signal Transduct Res. 1997 Jan-May;17(1-3):459-73. doi: 10.3109/10799899709036621. J Recept Signal Transduct Res. 1997. PMID: 9029508 Review.
-
Cooperative self-assembly of porphyrins with polymers possessing bioactive functions.Chem Commun (Camb). 2016 Nov 15;52(93):13543-13555. doi: 10.1039/c6cc05449h. Chem Commun (Camb). 2016. PMID: 27709187 Review.
Cited by
-
Duplex-forming oligocarbamates with tunable nonbonding sites.Chem Sci. 2024 May 13;15(24):9138-9146. doi: 10.1039/d4sc00242c. eCollection 2024 Jun 19. Chem Sci. 2024. PMID: 38903212 Free PMC article.
-
Assembly and Functionality of 2D Protein Arrays.Adv Sci (Weinh). 2025 Apr;12(15):e2416485. doi: 10.1002/advs.202416485. Epub 2025 Mar 16. Adv Sci (Weinh). 2025. PMID: 40089855 Free PMC article. Review.
-
Simplicity in the Design, Operation, and Applications of Mechanically Interlocked Molecular Machines.ACS Cent Sci. 2020 Feb 26;6(2):117-128. doi: 10.1021/acscentsci.9b01185. Epub 2020 Jan 22. ACS Cent Sci. 2020. PMID: 32123730 Free PMC article. Review.
-
Analysis of cooperativity by isothermal titration calorimetry.Int J Mol Sci. 2009 Aug 4;10(8):3457-77. doi: 10.3390/ijms10083457. Int J Mol Sci. 2009. PMID: 20111687 Free PMC article. Review.
-
Multivalence cooperativity leading to "all-or-nothing" assembly: the case of nucleation-growth in supramolecular polymers.Chem Sci. 2016 Jul 1;7(7):4468-4475. doi: 10.1039/c6sc00520a. Epub 2016 Mar 21. Chem Sci. 2016. PMID: 30009001 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

