Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability
- PMID: 16492807
- PMCID: PMC2063701
- DOI: 10.1083/jcb.200508154
Repo-Man recruits PP1 gamma to chromatin and is essential for cell viability
Abstract
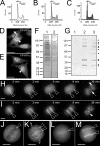
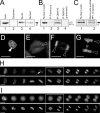
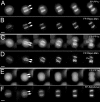
Protein phosphatase 1 (PP1) is a ubiquitous serine/threonine phosphatase regulating many cellular processes. PP1alpha and -gamma are closely related isoforms with distinct localization patterns, shown here by time-lapse microscopy of stably expressed fluorescent protein fusions. A pool of PP1gamma is selectively loaded onto chromatin at anaphase. Using stable isotope labeling and proteomics, we identified a novel PP1 binding protein, Repo-Man, which selectively recruits PP1gamma onto mitotic chromatin at anaphase and into the following interphase. This approach revealed both novel and known PP1 binding proteins, quantitating their relative distribution between PP1alpha and -gamma in vivo. When overexpressed, Repo-Man can also recruit PP1alpha to chromatin. Mutating Repo-Man's PP1 binding domain does not disrupt chromatin binding but abolishes recruitment of PP1 onto chromatin. RNA interference-induced knockdown of Repo-Man caused large-scale cell death by apoptosis, as did overexpression of this dominant-negative mutant. The data indicate that Repo-Man forms an essential complex with PP1gamma and is required for the recruitment of PP1 to chromatin.
Figures






References
-
- Allen, P.B., Y.G. Kwon, A.C. Nairn, and P. Greengard. 1998. Isolation and characterization of PNUTS, a putative protein phosphatase 1 nuclear targeting subunit. J. Biol. Chem. 273:4089–4095. - PubMed
-
- Andersen, J.S., C.E. Lyon, A.H. Fox, A.K. Leung, Y.W. Lam, H. Steen, M. Mann, and A.I. Lamond. 2002. Directed proteomic analysis of the human nucleolus. Curr. Biol. 12:1–11. - PubMed
-
- Andersen, J.S., Y.W. Lam, A.K. Leung, S.E. Ong, C.E. Lyon, A.I. Lamond, and M. Mann. 2005. Nucleolar proteome dynamics. Nature. 433:77–83. - PubMed
-
- Blagoev, B., S.E. Ong, I. Kratchmarova, and M. Mann. 2004. Temporal analysis of phosphotyrosine-dependent signaling networks by quantitative proteomics. Nat. Biotechnol. 22:1139–1145. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

