The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover
- PMID: 16492811
- PMCID: PMC2063702
- DOI: 10.1083/jcb.200512105
The Vac14p-Fig4p complex acts independently of Vac7p and couples PI3,5P2 synthesis and turnover
Abstract
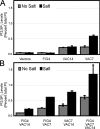
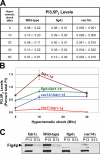
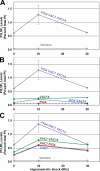
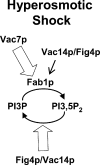
Phosphoinositide-signaling lipids function in diverse cellular pathways. Dynamic changes in the levels of these signaling lipids regulate multiple processes. In particular, when Saccharomyces cerevisiae cells are exposed to hyperosmotic shock, PI3,5P2 (phosphatidylinositol [PI] 3,5-bisphosphate) levels transiently increase 20-fold. This causes the vacuole to undergo multiple acute changes. Control of PI3,5P2 levels occurs through regulation of both its synthesis and turnover. Synthesis is catalyzed by the PI3P 5-kinase Fab1p, and turnover is catalyzed by the PI3,5P2 5-phosphatase Fig4p. In this study, we show that two putative Fab1p activators, Vac7p and Vac14p, independently regulate Fab1p activity. Although Vac7p only regulates Fab1p, surprisingly, we find that Vac14 regulates both Fab1p and Fig4p. Moreover, Fig4p itself functions in both PI3,5P2 synthesis and turnover. In both the absence and presence of Vac7p, the Vac14p-Fig4p complex controls the hyperosmotic shock-induced increase in PI3,5P2 levels. These findings suggest that the dynamic changes in PI3,5P2 are controlled through a tight coupling of synthesis and turnover.
Figures

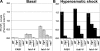


References
-
- Berwick, D.C., G.C. Dell, G.I. Welsh, K.J. Heesom, I. Hers, L.M. Fletcher, F.T. Cooke, and J.M. Tavare. 2004. Protein kinase B phosphorylation of PIKfyve regulates the trafficking of GLUT4 vesicles. J. Cell Sci. 117:5985–5993. - PubMed
-
- Blondeau, F., J. Laporte, S. Bodin, G. Superti-Furga, B. Payrastre, and J.L. Mandel. 2000. Myotubularin, a phosphatase deficient in myotubular myopathy, acts on phosphatidylinositol 3-kinase and phosphatidylinositol 3-phosphate pathway. Hum. Mol. Genet. 9:2223–2229. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

