M17, a gene specific for germinal center (GC) B cells and a prognostic marker for GC B-cell lymphomas, is dispensable for the GC reaction in mice
- PMID: 16493007
- PMCID: PMC1895815
- DOI: 10.1182/blood-2005-10-4154
M17, a gene specific for germinal center (GC) B cells and a prognostic marker for GC B-cell lymphomas, is dispensable for the GC reaction in mice
Abstract
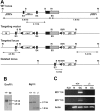
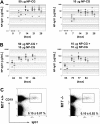
In T-cell-dependent antibody responses, antigen-specific B cells undergo a phase of secondary antibody diversification in germinal centers (GCs). Somatic hypermutation (SHM) introduces mutations into the rearranged immunoglobulin (Ig) variable (V) region genes, and class-switch recombination (CSR) alters the Ig heavy (H) chain constant region. Aberrant SHM or CSR is thought to contribute to the development of GC-derived B-cell malignancies. Diffuse large B-cell lymphomas (DLBCLs) are a heterogeneous group of such GC-derived tumors. Based on their gene expression profile, DLBCLs can be divided into activated B-cell-like and GC-like subgroups. The human gene HGAL is predominantly expressed in GCs. It is also part of the gene expression signature of GC-like DLBCL, and its high expression in DLBCL has been associated with a better clinical prognosis. We have generated mice deficient of the HGAL homologue M17 in order to investigate its functional significance. The mutant animals form normal GCs, undergo efficient CSR and SHM, and mount T-cell-dependent antibody responses similar to wild-type controls. Thus, M17 is dispensable for the GC reaction, and its potential function in the pathogenesis of DLBCL remains elusive.
Figures


References
-
- MacLennan IC. Germinal centers. Annu Rev Immunol. 1994;12: 117-139. - PubMed
-
- Tarlinton D. Germinal centers: form and function. Curr Opin Immunol. 1998;10: 245-251. - PubMed
-
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381: 751-758. - PubMed
-
- Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends Immunol. 2002;23: 31-39. - PubMed
-
- Chaudhuri J, Alt FW. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nat Rev Immunol. 2004;4: 541-552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

