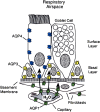
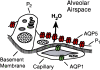
The aquaporin water channels
- PMID: 16493146
- PMCID: PMC2658677
- DOI: 10.1513/pats.200510-109JH
The aquaporin water channels
Figures


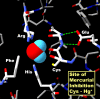
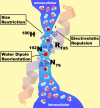
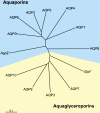






Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
