The role of biomarkers in clinical trials for Alzheimer disease
- PMID: 16493230
- PMCID: PMC1820855
- DOI: 10.1097/01.wad.0000191420.61260.a8
The role of biomarkers in clinical trials for Alzheimer disease
Abstract
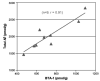
Biomarkers are likely to be important in the study of Alzheimer disease (AD) for a variety of reasons. A clinical diagnosis of Alzheimer disease is inaccurate even among experienced investigators in about 10% to 15% of cases, and biomarkers might improve the accuracy of diagnosis. Importantly for the development of putative disease-modifying drugs for Alzheimer disease, biomarkers might also serve as indirect measures of disease severity. When used in this way, sample sizes of clinical trials might be reduced, and a change in biomarker could be considered supporting evidence of disease modification. This review summarizes a meeting of the Alzheimer's Association's Research Roundtable, during which existing and emerging biomarkers for AD were evaluated. Imaging biomarkers including volumetric magnetic resonance imaging and positron emission tomography assessing either glucose utilization or ligands binding to amyloid plaque are discussed. Additionally, biochemical biomarkers in blood or cerebrospinal fluid are assessed. Currently appropriate uses of biomarkers in the study of Alzheimer disease, and areas where additional work is needed, are discussed.
Figures



References
-
- Matthews B, Siemers ER, Mozley PD. Imaging-based measures of disease progression in clinical trials of disease-modifying drugs for Alzheimer disease. Am J Geriatr Psychiatry. 2003;11:146–159. - PubMed
-
- Frank RA, Galasko D, Hampel H, et al. Biological markers for therapeutic trials in Alzheimer’s disease. Proceedings of the biological markers working group; NIA initiative on neuroimaging in Alzheimer’s disease. Neurobiol Aging. 2003;24:521–536. - PubMed
-
- Silbert LC, Quinn JF, Moore MM, et al. Changes in premorbid brain volume predict Alzheimer’s disease pathology. Neurology. 2003;61:487–492. - PubMed
-
- Fox NC, Cousens S, Scahill R, et al. Using serial registered brain magnetic resonance imaging to measure disease progression in Alzheimer disease: power calculations and estimates of sample size to detect treatment effects. Arch Neurol. 2000;57:339–344. - PubMed
-
- Alexander GE, Chen K, Pietrini P, et al. Longitudinal PET evaluation of cerebral metabolic decline in dementia: a potential outcome measure in Alzheimer’s disease treatment studies. Am J Psychiatry. 2002;159:738–745. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

