Engineered covalent leucotoxin heterodimers form functional pores: insights into S-F interactions
- PMID: 16494579
- PMCID: PMC1462717
- DOI: 10.1042/BJ20051878
Engineered covalent leucotoxin heterodimers form functional pores: insights into S-F interactions
Abstract
The staphylococcal alpha-toxin and bipartite leucotoxins belong to a single family of pore-forming toxins that are rich in beta-strands, although the stoichiometry and electrophysiological characteristics of their pores are different. The different known structures show a common beta-sandwich domain that plays a key role in subunit-subunit interactions, which could be targeted to inhibit oligomerization of these toxins. We used several cysteine mutants of both HlgA (gamma-haemolysin A) and HlgB (gamma-haemolysin B) to challenge 20 heterodimers linked by disulphide bridges. A new strategy was developed in order to obtain a good yield for S-S bond formation and dimer stabilization. Functions of the pores formed by 14 purified dimers were investigated on model membranes, i.e. planar lipid bilayers and large unilamellar vesicles, and on target cells, i.e. rabbit and human red blood cells and polymorphonuclear neutrophils. We observed that dimers HlgA T28C-HlgB N156C and HlgA T21C-HlgB T157C form pores with similar characteristics as the wild-type toxin, thus suggesting that the mutated residues are facing one another, allowing pore formation. Our results also confirm the octameric stoichiometry of the leucotoxin pores, as well as the parity of the two monomers in the pore. Correctly assembled heterodimers thus constitute the minimal functional unit of leucotoxins. We propose amino acids involved in interactions at one of the two interfaces for an assembled leucotoxin.
Figures

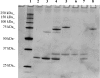




References
-
- Prévost G., Mourey L., Colin D. A., Monteil H., Dalla Serra M., Menestrina G. α-Toxin and β-barrel pore-forming toxins (leucocidins, α-, γ-, and δ-cytolysins) of Staphylococcus aureus. In: Alouf J. E., Popoff M. R., editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. London: Academic Press; 2005. pp. 588–605.
-
- Rossjohn J., Feil S. C., McKinstry W. J., Tweten R. K., Parker M. W. Structure of a cholesterol-binding, thiol-activated cytolysin and a model of its membrane form. Cell. 1997;89:685–692. - PubMed
-
- Moayeri M., Leppla S. H. The roles of anthrax toxin in pathogenesis. Curr. Opin. Microbiol. 2004;7:19–24. - PubMed
-
- Aktories K. Clostridial ADP-ribosylating toxins: effects on ATP and GTP-binding proteins. Mol. Cell. Biochem. 1994;138:167–176. - PubMed
-
- Thelestam M., Olofsson A., Blomqvist L., Hebert H. Oligomerization of cell-bound staphylococcal α-toxin in relation to membrane permeabilisation. Biochim. Biophys. Acta. 1991;1062:245–254. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

